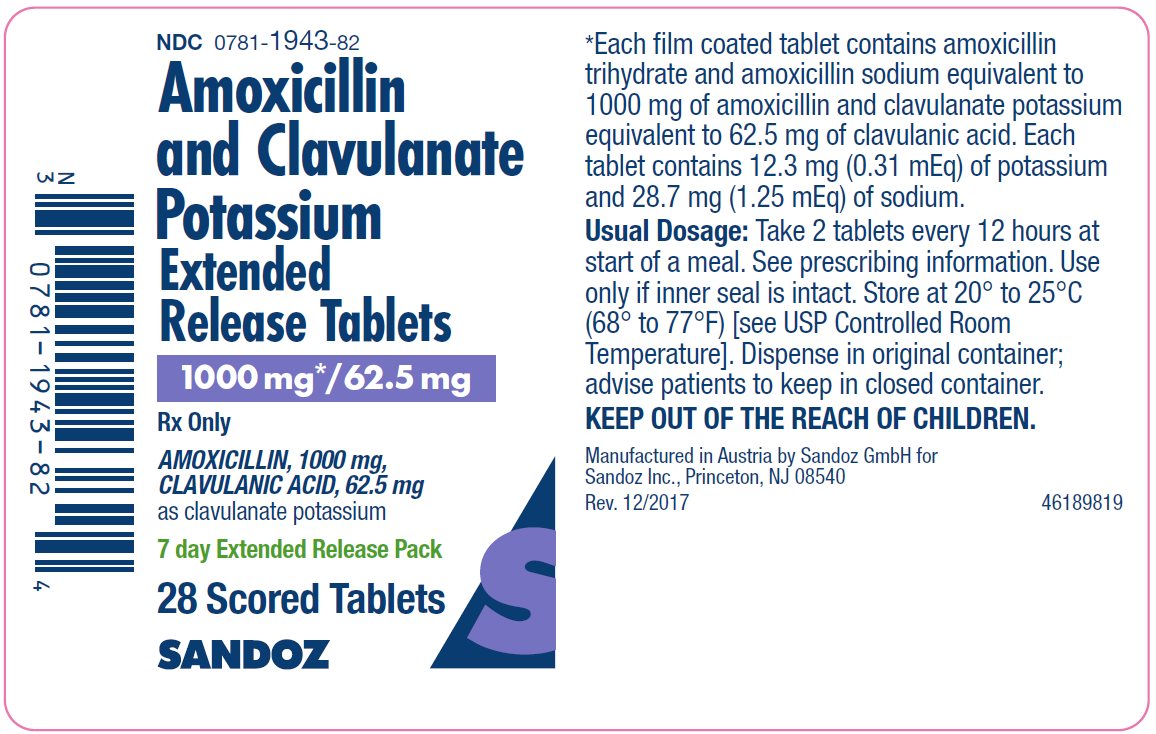
Label: AMOXICILLIN AND CLAVULANATE POTASSIUM tablet, multilayer, extended release
- NDC Code(s): 0781-1943-39, 0781-1943-82
- Packager: Sandoz Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AMOXICILLIN AND CLAVULANATE POTASSIUM EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for AMOXICILLIN AND CLAVULANATE POTASSIUM EXTENDED-RELEASE TABLETS.
AMOXICILLIN and CLAVULANATE POTASSIUM extended-release tablets, for oral use.
Initial U.S. Approval: 2002RECENT MAJOR CHANGES
- Warnings and Precautions (5) 11/2022
INDICATIONS AND USAGE
Amoxicillin and clavulanate potassium extended-release tablets is a combination of amoxicillin, a penicillin-class antibacterial and clavulanate potassium, a β-lactamase inhibitor, indicated for treatment of adults and pediatric patients with
- •
- community-acquired pneumonia or
- •
- acute bacterial sinusitis
- due to confirmed, or suspected β-lactamase-producing pathogens (i.e., H. influenzae, M. catarrhalis, H. parainfluenzae, K. pneumoniae, or methicillin-susceptible S. aureus) and S. pneumoniae with reduced susceptibility to penicillin (i.e., penicillin MICs equal to 2 mcg/mL). (1)
Limitations of Use
Amoxicillin and clavulanate potassium extended-release tablets is not indicated for the treatment of infections due to S. pneumoniae with penicillin MICs greater than or equal to 4 mcg/mL. Data are limited with regard to infections due to S. pneumoniae with penicillin MICs greater than or equal to 4 mcg/mL. (1)
Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of amoxicillin and clavulanate potassium extended-release tablets and other antibacterial drugs, amoxicillin and clavulanate potassium extended-release tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1)
DOSAGE AND ADMINISTRATION
- •
- Adults and Pediatric Patients >40 kg: The recommended dosage of amoxicillin and clavulanate potassium extended-release tablets is 4,000 mg/250 mg daily at the start of a meal according to the following table (2):
Indication
Dose
Duration
Acute bacterial sinusitis
2 tablets every 12 hours
10 days
Community-acquired pneumonia
2 tablets every 12 hours
7 to 10 days
DOSAGE FORMS AND STRENGTHS
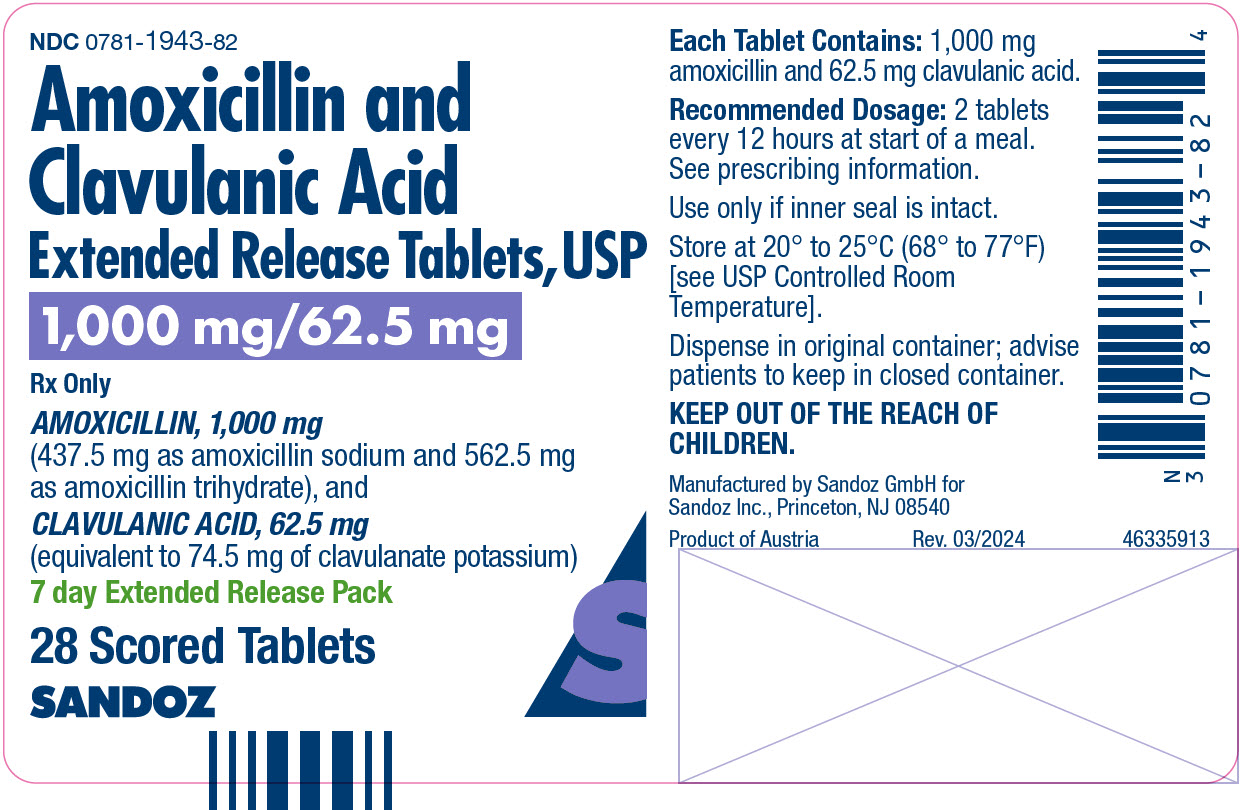
Extended-release Tablets: 1,000 mg/62.5 mg (3)
CONTRAINDICATIONS
- •
- History of a serious hypersensitivity reaction (e.g., anaphylaxis or Stevens-Johnson syndrome) to amoxicillin and clavulanate potassium extended-release tablets or to other β‑lactams (e.g., penicillins or cephalosporins) (4.1)
- •
- History of cholestatic jaundice/hepatic dysfunction associated with amoxicillin and clavulanate potassium extended-release tablets. (4.2)
- •
- In patients with severe renal impairment (creatinine clearance less than 30 mL/min) and in hemodialysis patients. (4.3)
WARNINGS AND PRECAUTIONS
- •
- Serious (including fatal) hypersensitivity reactions: Discontinue amoxicillin and clavulanate potassium extended-release tablets if a reaction occurs. (5.1)
- •
- Severe cutaneous adverse reactions (SCAR): Monitor closely. Discontinue if rash progresses. (5.2)
- •
- Hepatic dysfunction and cholestatic jaundice: Discontinue if signs/symptoms of hepatitis occur. Monitor liver function tests in patients with hepatic impairment. (5.3)
- •
- Clostridioides difficile-associated diarrhea (CDAD): Evaluate patients if diarrhea occurs. (5.4)
- •
- Patients with mononucleosis who receive amoxicillin and clavulanate potassium extended-release tablets develop skin rash. Avoid amoxicillin and clavulanate potassium extended-release tablets use in these patients. (5.5)
ADVERSE REACTIONS
The most frequently reported adverse reactions were diarrhea (15%), vaginal mycosis (3%) nausea (2%), and loose stools (2%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc. at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Co-administration with probenecid is not recommended. (7.1)
- •
- Concomitant use of amoxicillin and clavulanate potassium extended-release tablets and oral anticoagulants may increase the prolongation of prothrombin time. (7.2)
- •
- Co-administration with allopurinol increases the risk of rash. (7.3)
- •
- Amoxicillin and clavulanate potassium extended-release tablets may reduce efficacy of oral contraceptives. (7.4)
USE IN SPECIFIC POPULATIONS
- •
- Renal Impairment: Amoxicillin and clavulanate potassium extended-release tablets have not been studied in patients with renal impairment. (8.6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosage in Adult Patients
2.3 Dosage in Pediatric Patients
2.5 Dosage in Patients with Hepatic Impairment
2.7 Switching between Dosage Forms and between Strengths
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Serious Hypersensitivity Reactions
4.2 Cholestatic Jaundice/Hepatic Dysfunction
4.3 Renal Impairment
5 WARNINGS AND PRECAUTIONS
5.1 Serious Allergic Reactions, Including Anaphylaxis
5.2 Severe Cutaneous Adverse Reactions
5.3 Hepatic Dysfunction
5.4 Clostridioides difficile-Associated Diarrhea (CDAD)
5.5 Skin Rash in Patients with Mononucleosis
5.6 Potential for Microbial Overgrowth
5.7 Development of Drug-Resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Probenecid
7.2 Oral Anticoagulants
7.3 Allopurinol
7.4 Oral Contraceptives
7.5 Effects on Laboratory Tests
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Acute Bacterial Sinusitis
14.2 Community-Acquired Pneumonia
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Amoxicillin and clavulanate potassium extended-release tablets is indicated for the treatment of infections in adults and pediatric patients with
- •
- community-acquired pneumonia or
- •
- acute bacterial sinusitis
due to confirmed, or suspected β-lactamase-producing pathogens (i.e., H. influenzae, M. catarrhalis, H. parainfluenzae, K. pneumoniae, or methicillin-susceptible S. aureus) and S. pneumoniae with reduced susceptibility to penicillin (i.e., penicillin MICs equal to 2 mcg/mL).
Limitations of Use
Amoxicillin and clavulanate potassium extended-release tablets is not indicated for the treatment of infections due to S. pneumoniae with penicillin MICs greater than or equal to 4 mcg/mL. Data are limited with regard to infections due to S. pneumoniae with penicillin MICs greater than or equal to 4 mcg/mL [see Clinical Studies (14)].
Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of amoxicillin and clavulanate potassium extended-release tablets and other antibacterial drugs, amoxicillin and clavulanate potassium extended-release tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
In patients with community-acquired pneumonia in whom penicillin-resistant S. pneumoniae is suspected, bacteriological studies should be performed to determine the causative organisms and their susceptibility when amoxicillin and clavulanate potassium extended-release tablets are prescribed.
Acute bacterial sinusitis or community-acquired pneumonia due to a penicillin-susceptible strain of S. pneumoniae plus a β-lactamase-producing pathogen can be treated with another amoxicillin and clavulanate potassium product containing lower daily doses of amoxicillin (i.e., 500 mg every 8 hours or 875 mg every 12 hours). Acute bacterial sinusitis or community-acquired pneumonia due to S. pneumoniae alone can be treated with amoxicillin.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Amoxicillin and clavulanate potassium extended-release tablets should be taken at the start of a meal to enhance the absorption of amoxicillin and to minimize the potential for gastrointestinal intolerance. Amoxicillin and clavulanate potassium extended-release tablets is not recommended to be taken with a high-fat meal because clavulanate absorption is decreased [see Clinical Pharmacology (12.3)].
2.2 Dosage in Adult Patients
The recommended dosage of amoxicillin and clavulanate potassium extended-release tablets is 4,000 mg/250 mg daily according to the following table:
Table 1: Recommended Dosage of Amoxicillin and Clavulanate Potassium Extended-Release Tablets in Adult Patients
Indication
Dose
Duration
Acute bacterial sinusitis
Two (1,000 mg/62.5 mg)
tablets every 12 hours
10 days
Community-acquired pneumonia
Two (1,000 mg/62.5 mg)
tablets every 12 hours
7 to 10 days
Amoxicillin and clavulanate potassium extended-release tablets can be split in half along the score line for patients with difficulty swallowing the tablets whole. Both halves of the tablet must be taken immediately.
2.3 Dosage in Pediatric Patients
Pediatric patients who weigh 40 kg or more and can swallow tablets should receive the adult dose [see Dosage and Administration (2.2) and Use in Specific Populations (8.4)].
2.5 Dosage in Patients with Hepatic Impairment
Hepatically impaired patients should be dosed with caution and hepatic function monitored at regular intervals [see Warnings and Precautions (5.3)].
2.7 Switching between Dosage Forms and between Strengths
Amoxicillin and clavulanate potassium extended-release tablet is NOT substitutable on a mg-to-mg basis with other formulations of amoxicillin and clavulanate potassium. In addition, the extended-release tablets provide an extended time course of plasma amoxicillin concentrations compared to immediate-release tablets. Thus, two amoxicillin and clavulanate potassium 500 mg tablets are not equivalent to one amoxicillin and clavulanate potassium extended-release 1,000 mg tablet.
-
3 DOSAGE FORMS AND STRENGTHS
Amoxicillin and Clavulanate Potassium Extended-Release Tablets:
- •
- 1,000 mg/62.5 mg: Each white to cream tinged, oval film-coated bilayer tablet, debossed SZ 137 on one side and scored on the reverse side, contains amoxicillin trihydrate and amoxicillin sodium equivalent to a total of 1,000 mg of amoxicillin and clavulanate potassium equivalent to 62.5 mg of clavulanic acid.
-
4 CONTRAINDICATIONS
4.1 Serious Hypersensitivity Reactions
Amoxicillin and clavulanate potassium extended-release tablets is contraindicated in patients with a history of serious hypersensitivity reactions (e.g., anaphylaxis or Stevens-Johnson syndrome) to amoxicillin, clavulanate or to other β-lactam antibacterial drugs (e.g., penicillins and cephalosporins).
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Allergic Reactions, Including Anaphylaxis
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving amoxicillin and clavulanate potassium extended-release tablets. These reactions are more likely to occur in individuals with a history of penicillin hypersensitivity and/or a history of sensitivity to multiple allergens. Before initiating therapy with amoxicillin and clavulanate potassium extended-release tablets, careful inquiry should be made regarding previous hypersensitivity reactions to penicillins, cephalosporins, or other allergens. If an allergic reaction occurs, discontinue amoxicillin and clavulanate potassium extended-release tablets and institute appropriate therapy.
5.2 Severe Cutaneous Adverse Reactions
Amoxicillin and clavulanate potassium extended-release tablets may cause severe cutaneous adverse reactions (SCAR), such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP). If patients develop a skin rash, they should be monitored closely, and amoxicillin and clavulanate potassium extended-release tablets discontinued if lesions progress.
5.3 Hepatic Dysfunction
Use amoxicillin and clavulanate potassium extended-release tablets with caution in patients with evidence of hepatic dysfunction. Hepatic toxicity associated with the use of amoxicillin and clavulanate potassium extended-release tablets is usually reversible. Deaths have been reported (fewer than one death reported per estimated four million prescriptions worldwide). These have generally been cases associated with serious underlying diseases or concomitant medications [see Contraindications (4.2), and Adverse Reactions (6.2)].
5.4 Clostridioides difficile-Associated Diarrhea (CDAD)
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including amoxicillin and clavulanate potassium extended- release tablets, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C.difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.5 Skin Rash in Patients with Mononucleosis
A high percentage of patients with mononucleosis who receive amoxicillin develop an erythematous skin rash. Thus, amoxicillin and clavulanate potassium extended-release tablets should not be administered to patients with mononucleosis.
5.6 Potential for Microbial Overgrowth
The possibility of superinfections with mycotic or bacterial pathogens should be kept in mind during therapy. If superinfections occur (usually involving Pseudomonas spp. or Candida spp.), the drug should be discontinued and/or appropriate therapy instituted.
5.7 Development of Drug-Resistant Bacteria
Prescribing amoxicillin and clavulanate potassium extended-release tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
-
6 ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
- •
- Anaphylactic reactions [see Warnings and Precautions (5.1)]
- •
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.2)]
- •
- Hepatic Dysfunction [see Warnings and Precautions (5.3)]
- •
- Clostridioides difficile-associated diarrhea (CDAD) [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials, 5,643 patients have been treated with amoxicillin and clavulanate potassium extended-release tablets. The most frequently reported adverse reactions which were suspected or probably drug- related were diarrhea (15%), vaginal mycosis (3%) nausea (2%), and loose stools (2%). Amoxicillin and clavulanate potassium extended-release tablets had a higher rate of diarrhea which required corrective therapy (4% versus 3% for amoxicillin and clavulanate potassium extended-release tablets and all comparators, respectively). Two percent of patients discontinued therapy because of drug-related adverse reactions.
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following have been identified during post-marketing use of amoxicillin and clavulanate potassium products, including amoxicillin and clavulanate potassium extended-release tablets. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to amoxicillin and clavulanate potassium.
Gastrointestinal: Diarrhea, nausea, vomiting, indigestion, gastritis, stomatitis, glossitis, black “hairy” tongue, mucocutaneous candidiasis, enterocolitis, and hemorrhagic/pseudo membranous colitis. Onset of pseudo membranous colitis symptoms may occur during or after antibacterial treatment [see Warnings and Precautions (5.4)].
Immune: Hypersensitivity reactions, anaphylactic/anaphylactoid reactions (including shock), angioedema, serum sickness-like reactions (urticaria or skin rash accompanied by arthritis, arthralgia, myalgia, and frequently fever), hypersensitivity vasculitis [see Warnings and Precautions (5.1)].
Skin and Appendages: Rashes, pruritus, urticaria, erythema multiforme, SJS, TEN, DRESS, AGEP, exfoliative dermatitis [see Warnings and Precautions (5.2)].
Liver: A moderate rise in AST (SGOT) and/or ALT (SGPT) has been noted in patients treated with ampicillin-class antibacterials, but the significance of these findings is unknown. Hepatic dysfunction, including hepatitis and cholestatic jaundice, [see Contraindications (4.2)], increases in serum transaminases (AST and/or ALT), serum bilirubin, and/or alkaline phosphatase, has been reported with amoxicillin and clavulanate potassium or amoxicillin and clavulanate potassium extended-release tablets. It has been reported more commonly in the elderly, in males, or in patients on prolonged treatment. The histologic findings on liver biopsy have consisted of predominantly cholestatic, hepatocellular, or mixed cholestatic-hepatocellular changes. The onset of signs/symptoms of hepatic dysfunction may occur during or several weeks after therapy has been discontinued. The hepatic dysfunction, which may be severe, is usually reversible. Deaths have been reported [see Contraindications (4.2), Warnings and Precautions (5.3)].
Renal: Interstitial nephritis, hematuria, and crystalluria have been reported [see Overdosage (10)].
Hemic and Lymphatic Systems: Anemia, including hemolytic anemia, thrombocytopenia, thrombocytopenic purpura, eosinophilia, leukopenia, and agranulocytosis have been reported during therapy with penicillins. These reactions are usually reversible on discontinuation of therapy and are believed to be hypersensitivity phenomena. There have been reports of increased prothrombin time in patients receiving amoxicillin and clavulanate potassium and anticoagulant therapy concomitantly.
Central Nervous System: Agitation, anxiety, behavioral changes, aseptic meningitis, confusion, convulsions, dizziness, headache, insomnia, and reversible hyperactivity have been reported.
Miscellaneous: Tooth discoloration (brown, yellow, or gray staining) has been reported. Most reports occurred in pediatric patients. Discoloration was reduced or eliminated with brushing or dental cleaning in most cases.
-
7 DRUG INTERACTIONS
7.1 Probenecid
Probenecid decreases the renal tubular secretion of amoxicillin. Concurrent use with amoxicillin and clavulanate potassium extended-release tablets may result in increased and prolonged blood levels of amoxicillin. Co-administration of probenecid is not recommended.
7.2 Oral Anticoagulants
Abnormal prolongation of prothrombin time (increased international normalized ratio [INR]) has been reported in patients receiving amoxicillin and oral anticoagulants. Appropriate monitoring should be undertaken when anticoagulants are prescribed concurrently. Adjustments in the dose of oral anticoagulants may be necessary to maintain the desired level of anticoagulation.
7.3 Allopurinol
The concurrent administration of allopurinol and amoxicillin substantially increases the incidence of rashes in patients receiving both drugs as compared to patients receiving amoxicillin alone. It is not known whether this potentiation of amoxicillin rashes is due to allopurinol or the hyperuricemia present in these patients. In controlled clinical trials of amoxicillin and clavulanate potassium extended-release tablets, 25 patients received concomitant allopurinol and amoxicillin and clavulanate potassium extended-release tablets. No rashes were reported in these patients. However, this sample size is too small to allow for any conclusions to be drawn regarding the risk of rashes with concomitant amoxicillin and clavulanate potassium extended-release tablets and allopurinol use.
7.4 Oral Contraceptives
Amoxicillin and clavulanate potassium extended-release tablets may affect intestinal flora, leading to lower estrogen reabsorption and reduced efficacy of combined oral estrogen/progesterone contraceptives.
7.5 Effects on Laboratory Tests
High urine concentrations of amoxicillin may result in false-positive reactions when testing for the presence of glucose in urine using CLINITEST®, Benedict’s Solution, or Fehling’s Solution. Since this effect may also occur with amoxicillin and clavulanate potassium extended release tablets, it is recommended that glucose tests based on enzymatic glucose oxidase reactions be used.
Following administration of amoxicillin to pregnant women, a transient decrease in plasma concentration of total conjugated estriol, estriol-glucuronide, conjugated estrone, and estradiol has been noted
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects
Reproduction studies performed in pregnant rats and mice given amoxicillin and clavulanate potassium at oral doses up to 1,200 mg/kg/day revealed no evidence of harm to the fetus due to amoxicillin and clavulanate potassium. In terms of body surface area, the doses in rats were 1.6 times the maximum human oral dose of amoxicillin and 13 times the maximum human dose for clavulanate. For mice, these doses were 0.9 and 7.4 times the maximum human oral dose of amoxicillin and clavulanate, respectively. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
8.2 Labor and Delivery
Oral ampicillin is poorly absorbed during labor. Studies in guinea pigs have shown that intravenous administration of ampicillin decreased the uterine tone, frequency of contractions, height of contractions, and duration of contractions. However, it is not known whether the use of amoxicillin and clavulanate potassium extended-release tablets in humans during labor or delivery has immediate or delayed adverse effects on the fetus, prolongs the duration of labor, or increases the likelihood that forceps delivery or other obstetrical intervention or resuscitation of the newborn will be necessary. In a single study in women with premature rupture of fetal membranes, it was reported that prophylactic treatment with amoxicillin and clavulanate potassium may be associated with an increased risk of necrotizing enterocolitis in neonates.
8.3 Nursing Mothers
Amoxicillin has been shown to be excreted in human milk; therefore, caution should be exercised when amoxicillin and clavulanate potassium extended-release tablets is administered to a nursing woman.
8.4 Pediatric Use
The safety and effectiveness of amoxicillin and clavulanate potassium extended-release tablets have been established for pediatric patients weighing greater than or equal to 40 kg who are able to swallow tablets. Use of amoxicillin and clavulanate potassium extended-release tablets in these pediatric patients is supported by evidence from adequate and well-controlled trials of adults with acute bacterial sinusitis and community-acquired pneumonia with additional data from a pediatric pharmacokinetic study.
A pharmacokinetic study in pediatric patients (7 to 15 years of age and weighing greater than or equal to 40 kg) was conducted [see Clinical Pharmacology (12.3)].
The adverse event profile in 44 pediatric patients who received at least one dose of amoxicillin and clavulanate potassium extended-release tablets was consistent with the established adverse event profile for the product in adults.
8.5 Geriatric Use
Of the total number of subjects in clinical studies of amoxicillin and clavulanate potassium extended-release tablets, 18% were 65 years or older and 7% were 75 years or older. No overall differences in safety and effectiveness were observed between these subjects and younger subjects, and other clinical experience has not reported differences in responses between the elderly and younger patients, but a greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of dose dependent toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function.
8.6 Renal Impairment
The pharmacokinetics of amoxicillin and clavulanate potassium extended-release tablets have not been studied in patients with renal impairment. Amoxicillin and clavulanate potassium extended-release tablets is contraindicated in patients with a creatinine clearance of less than 30 mL/min and in hemodialysis patients [see Contraindications (4.3)].
-
10 OVERDOSAGE
Following overdosage, patients have experienced primarily gastrointestinal symptoms including stomach and abdominal pain, vomiting, and diarrhea. Rash, hyperactivity, or drowsiness have also been observed in a small number of patients.
In the case of overdosage, discontinue amoxicillin and clavulanate potassium extended-release tablets, treat symptomatically, and institute supportive measures as required. If the overdosage is very recent and there is no contraindication, an attempt at emesis or other means of removal of drug from the stomach may be performed. A prospective study of 51 pediatric patients at a poison control center suggested that overdosages of less than 250 mg/kg of amoxicillin are not associated with significant clinical symptoms and do not require gastric emptying1.
Interstitial nephritis resulting in oliguric renal failure has been reported in a small number of patients after overdosage with amoxicillin.
Crystalluria, in some cases leading to renal failure, has also been reported after amoxicillin overdosage in adult and pediatric patients. In the case of overdosage, adequate fluid intake and diuresis should be maintained to reduce the risk of amoxicillin crystalluria.
Renal impairment appears to be reversible with cessation of drug administration. High blood levels may occur more readily in patients with impaired renal function because of decreased renal clearance of both amoxicillin and clavulanate. Both amoxicillin and clavulanate are removed from the circulation by hemodialysis.
-
11 DESCRIPTION
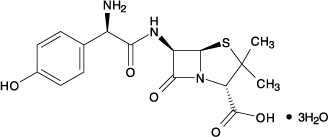
Amoxicillin and clavulanate potassium extended-release tablets for oral use is an antibacterial combination consisting of the semisynthetic antibacterial amoxicillin (present as amoxicillin trihydrate and amoxicillin sodium) and the β-lactamase inhibitor clavulanate potassium (the potassium salt of clavulanic acid). Amoxicillin is an analog of ampicillin, derived from the basic penicillin nucleus, 6-aminopenicillanic acid. The amoxicillin trihydrate molecular formula is C16H19N3O5S•3H2O, and the molecular weight is 419.45. Chemically, amoxicillin trihydrate is (2S,5R,6R)-6-[(R)-(-)-2-Amino-2-(p-hydroxyphenyl)acetamido]-3,3‑ dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid trihydrate and may be represented structurally as:
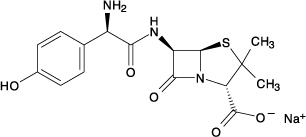
The amoxicillin sodium molecular formula is C16H18N3NaO5S, and the molecular weight is 387.39. Chemically, amoxicillin sodium is [2 -[2α,5α,6β(S*)]]-6-[[Amino(4‑ hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1- azabicyclo[3.2.0]heptane-2‑ carboxylic acid monosodium salt and may be represented structurally as:
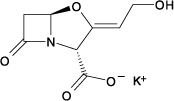
Clavulanic acid is produced by the fermentation of Streptomyces clavuligerus. It is a β‑lactam structurally related to the penicillins and possesses the ability to inactivate a wide variety of β-lactamases by blocking the active sites of these enzymes. Clavulanic acid is particularly active against the clinically important plasmid-mediated β-lactamases frequently responsible for transferred drug resistance to penicillins and cephalosporins. The clavulanate potassium molecular formula is C8H8KNO5, and the molecular weight is 237.25. Chemically, clavulanate potassium is potassium (Z)-(2R,5R)-3-(2-hydroxy ethylidene)-7-oxo-4-oxa-1-azabicyclo[3.2.0]-heptane-2-carboxylate, and may be represented structurally as:
Each tablet of amoxicillin and clavulanate potassium extended-release contains 1,000 mg of amoxicillin (437.5 mg as amoxicillin sodium and 562.5 mg as amoxicillin trihydrate), and 62.5 mg of clavulanic acid (equivalent to 74.5 mg of clavulanate potassium).
Inactive Ingredients: Anhydrous citric acid, colloidal silicon dioxide, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sodium starch glycolate, titanium dioxide, and xanthan gum.
Each tablet of amoxicillin and clavulanate potassium extended-release tablets contains approximately 12 mg of potassium and 29 mg of sodium.
Meets USP Dissolution Test 2.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Amoxicillin and clavulanate potassium extended-release tablets is an antibacterial drug [see Microbiology (12.4)].
12.3 Pharmacokinetics
Amoxicillin and clavulanate potassium extended-release tablets is an extended-release formulation which provides sustained plasma concentrations of amoxicillin. Amoxicillin systemic exposure achieved with amoxicillin and clavulanate potassium extended-release tablets is similar to that produced by the oral administration of equivalent doses of amoxicillin alone.
Absorption
Amoxicillin and clavulanate potassium are well absorbed from the gastrointestinal tract after oral administration of amoxicillin and clavulanate potassium extended-release tablets.
In a study of healthy adult volunteers, the pharmacokinetics of amoxicillin and clavulanate potassium extended-release tablets were compared when administered in a fasted state, at the start of a standardized meal (612 kcal, 89.3 g carb, 24.9 g fat, and 14 g protein), or 30 minutes after a high‑fat meal. When the systemic exposure to both amoxicillin and clavulanate is taken into consideration, amoxicillin and clavulanate potassium extended-release tablets is optimally administered at the start of a standardized meal. Absorption of amoxicillin is decreased in the fasted state. Amoxicillin and clavulanate potassium extended-release tablets is not recommended to be taken with a high‑fat meal, because clavulanate absorption is decreased. The pharmacokinetics of the components of amoxicillin and clavulanate potassium extended-release tablets following administration of two amoxicillin and clavulanate potassium extended-release tablets at the start of a standardized meal are presented in Table 2.
Table 2: Mean (SD) Pharmacokinetic Parameter for Amoxicillin and Clavulanate Following Oral Administration of Two Amoxicillin and Clavulanate Potassium Extended-Release Tablets (2,000 mg/125 mg) to Healthy Adult Volunteers (n = 55) Fed a Standardized Meal
Parameter (units)
Amoxicillin
Clavulanate
AUC(0‑inf) (mcg•hr/mL)
71.6 (16.5)
5.29 (1.55)
Cmax (mcg/mL)
17.0 (4.0)
2.05 (0.80)
Tmax (hours)*
1.50 (1.00 ‑ 6.00)
1.03 (0.75 ‑ 3.00)
T1/2 (hours)
1.27 (0.20)
1.03 (0.17)
* Median (range).
The half-life of amoxicillin after the oral administration of amoxicillin and clavulanate potassium extended-release tablets is approximately 1.3 hours, and that of clavulanate is approximately 1.0 hour.
Distribution
Neither component in amoxicillin and clavulanate potassium extended-release tablets is highly protein‑bound; clavulanate has been found to be approximately 25% bound to human serum and amoxicillin approximately 18% bound.
Amoxicillin diffuses readily into most body tissues and fluids, with the exception of the brain and spinal fluid. The results of experiments involving the administration of clavulanic acid to animals suggest that this compound, like amoxicillin, is well distributed in body tissues.
Excretion
Clearance of amoxicillin is predominantly renal, with approximately 60% to 80% of the dose being excreted unchanged in urine, whereas clearance of clavulanate has both a renal (30% to 50%) and a non‑renal component.
Drug Interactions
Concurrent administration of probenecid delays amoxicillin excretion but does not delay renal excretion of clavulanate [see Drug Interactions (7.1)].
In a study of adults, the pharmacokinetics of amoxicillin and clavulanate were not affected by administration of an antacid (MAALOX®), either simultaneously with or 2 hours after amoxicillin and clavulanate potassium extended-release tablets.
Pediatrics
In a study of pediatric patients with acute bacterial sinusitis, 7 to 15 years of age, and weighing at least 40 kg, the pharmacokinetics of amoxicillin and clavulanate were assessed following administration of amoxicillin and clavulanate potassium extended-release tablets 2000 mg/125 mg (as two 1000 mg/62.5 mg tablets) every 12 hours with food (Table 3).
Table 3: Mean (SD) Pharmacokinetic Parameters for Amoxicillin and Clavulanate Following Oral Administration of Two Amoxicillin and Clavulanate Potassium Extended-Release Tablets (2,000 mg/125 mg) Every 12 Hours with Food to Pediatric Patients (7 to 15 Years of Age and Weighing ≥ 40kg) With Acute Bacterial Sinusitis
Parameter (units)
Amoxicillin
(n=24)
Clavulanate
(n=23)
AUC(0‑τ) (mcg•hr/mL)
57.8 (15.6)
3.18 (1.37)
Cmax (mcg/mL)
11.0 (3.34)
1.17 (0.67)
Tmax (hours)
2.0 (1.0 - 5.0)
2.0 (1.0 - 4.0)
T1/2(hours)
3.32 (2.21)†
0.94 (0.13)‡
* Median (range).
† n=18.
‡ n=17.
12.4 Microbiology
Mechanism of Action
Amoxicillin binds to penicillin-binding proteins within the bacterial cell wall and inhibits bacterial cell wall synthesis.
Clavulanic acid is a β-lactam, structurally related to penicillin, that may inactivate certain β‑lactamase enzymes.
Resistance
Resistance to penicillins may be mediated by destruction of the β-lactam ring by a β-lactamase, altered affinity of penicillin for target, or decreased penetration of the antibacterial drug to reach the target site. Amoxicillin alone is susceptible to degradation by β‑lactamases, and therefore its spectrum of activity does not include bacteria that produce these enzymes.
Antimicrobial Activity
Amoxicillin/clavulanic acid has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1)].
Gram-positive Bacteria:
Staphylococcus aureus (methicillin-susceptible)
Streptococcus pneumoniae
Gram-negative Bacteria:
Haemophilus influenzae
Haemophilus parainfluenzae
Klebsiella pneumoniae
Moraxella catarrhalis
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for amoxicillin and clavulanic acid against isolates of similar genus or organism group. However, the efficacy of amoxicillin and clavulanic acid in treating clinical infections caused by these bacteria have not been established in adequate and well-controlled clinical trials.
Gram-positive bacteria:
Streptococcus pyogenes
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long‑term studies in animals have not been performed to evaluate carcinogenic potential. The mutagenic potential of amoxicillin and clavulanate potassium was investigated in vitro with an Ames test, a human lymphocyte cytogenetic assay, a yeast test, and a mouse lymphoma forward mutation assay, and in vivo with mouse micronucleus tests and a dominant lethal test. All were negative apart from the in vitro mouse lymphoma assay, where weak activity was found at very high, cytotoxic concentrations. Amoxicillin and clavulanate potassium at oral doses of up to 1,200 mg/kg/day (1.9 times the maximum human dose of amoxicillin and 15 times the maximum human dose of clavulanate based on body surface area) was found to have no effect on fertility and reproductive performance in rats dosed with a 2:1 ratio formulation of amoxicillin:clavulanate.
-
14 CLINICAL STUDIES
14.1 Acute Bacterial Sinusitis
Adults with a diagnosis of acute bacterial sinusitis (ABS) were evaluated in 3 clinical studies. In one study, 363 patients were randomized to receive either amoxicillin and clavulanate potassium extended-release tablets 2,000 mg/125 mg orally every 12 hours or levofloxacin 500 mg orally daily for 10 days in a double‑blind, multicenter, prospective trial. These patients were clinically and radiologically evaluated at the test of cure (day 17 to 28) visit. The combined clinical and radiological responses were 84% for amoxicillin and clavulanate potassium extended-release tablets and 84% for levofloxacin at the test of cure visit in clinically evaluable patients (95% CI for the treatment difference equals ‑9.4, 8.3). The clinical response rates at the test of cure were 87% and 89%, respectively.
The other 2 trials were non‑comparative, multicenter studies designed to assess the bacteriological and clinical efficacy of amoxicillin and clavulanate potassium extended-release tablets (2,000 mg/125 mg orally every 12 hours for 10 days) in the treatment of 2,288 patients with ABS. Evaluation time points were the same as in the prior study. Patients underwent maxillary sinus puncture for culture prior to receiving study medication. Patients with acute bacterial sinusitis due to S.pneumoniae with reduced susceptibility to penicillin were accrued through enrollment in these 2 open‑label non‑comparative clinical trials. Microbiologic eradication rates for key pathogens in these studies are shown in Table 4.
Table 4: Clinical Outcome for ABS
Penicillin MICs of S. pneumoniae Isolates
Intent-To-Treat
Clinically Evaluable
n/N
%
95% CI†
n/N
%
95% CI†
All S. pneumoniae
344/370
93
—
318/326
98
—
MIC ≥ 2.0 mcg/mL‡
35/36
97
85.5, 99.9
30/31
96
83.3, 99.9
MIC = 2.0 mcg/mL
23/24
96
78.9, 99.9
19/20
95
75.1, 99.9
MIC ≥ 4.0 mcg/mL§
12/12
100
73.5, 100
11/11
100
71.5, 100
H. influenzae
265/305
87
—
242/259
93
—
M. catarrhalis
94/105
90
—
86/90
96
—
* n/N = patients with pathogen eradicated or presumed eradicated/total number of patients.
† Confidence limits calculated using exact probabilities.
‡ S. pneumoniae strains with penicillin MICs of ≥ 2 mcg/mL are considered resistant to penicillin.
§ Includes one patient each with S. pneumoniae penicillin MICs of 8 and 16 mcg/mL.
14.2 Community-Acquired Pneumonia
Four randomized, controlled, double‑blind clinical studies and one non‑comparative study were conducted in adults with community-acquired pneumonia (CAP). In comparative studies, 904 patients received amoxicillin and clavulanate potassium extended-release tablets at a dose of 2,000 mg/125 mg orally every 12 hours for 7 or 10 days. In the non-comparative study to assess both clinical and bacteriological efficacy, 1,122 patients received amoxicillin and clavulanate potassium extended-release tablets 2,000 mg/125 mg orally every 12 hours for 7 days. In the 4 comparative studies, the combined clinical success rate at test of cure ranged from 86% to 95% in clinically evaluable patients who received amoxicillin and clavulanate potassium extended-release tablets.
Data on the efficacy of amoxicillin and clavulanate potassium extended-release tablets in the treatment of community‑ acquired pneumonia due to S.pneumoniae with reduced susceptibility to penicillin were accrued from the 4 controlled clinical studies and the 1 non‑comparative study. The majority of these cases were accrued from the non‑comparative study. Results are shown in Table 5.
Table 5: Clinical Outcome for CAP due to S.pneumoniae
Penicillin MICs of S. pneumoniae Isolates
Intent-To-Treat
Clinically Evaluable
n/N
%
95% CI†
n/N
%
95% CI†
All S. pneumoniae
318/367
87
____
275/297
93
_____
MIC ≥ 2.0 mcg/mL‡
30/35
86
69.7, 95.2
24/25
96
79.6, 99.9
MIC = 2.0 mcg/mL
22/24
92
73.0, 99.0
18/18
100
81.5, 100
MIC ≥ 4.0 mcg/mL§
8/11
73
39.0,94.0
6/7
86
42.1, 99.6
* n/N = patients with pathogen eradicated or presumed eradicated/total number of patients.
† Confidence limits calculated using exact probabilities.
‡ S. pneumoniae strains with penicillin MICs of ≥ 2 mcg/mL are considered resistant to penicillin.
§ Includes one patient each with S. pneumoniae penicillin MICs of 8 and 16 mcg/mL in the Intent‑To‑Treat group only.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Amoxicillin and Clavulanate Potassium Extended-Release Tablets: Each white to cream tinged, oval film‑coated bilayer tablet, debossed SZ 137 on one side and scored on the reverse side, contains amoxicillin trihydrate and amoxicillin sodium equivalent to a total of 1,000 mg of amoxicillin and clavulanate potassium equivalent to 62.5 mg of clavulanic acid.
NDC 0781-1943-82 Bottles of 28 (7 day Extended Release Tablets pack)
NDC 0781-1943-39 Bottles of 40 (10 day Extended Release Tablets pack)
Storage
Store tablets at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense in original container.
KEEP OUT OF THE REACH OF CHILDREN.
-
17 PATIENT COUNSELING INFORMATION
Administration Instructions
Counsel patients to take amoxicillin and clavulanate potassium extended-release tablets every 12 hours with a low fat meal or snack to reduce the possibility of gastrointestinal upset. If diarrhea develops and is severe or lasts more than 2 or 3 days, call your doctor.
Allergic Reactions
Counsel patients that amoxicillin and clavulanate potassium extended-release tablets contains a penicillin class drug product that can cause allergic reactions in some individuals.
Severe Cutaneous Adverse Reactions (SCAR)
Advise patients about the signs and symptoms of serious skin manifestations. Instruct patients to stop taking amoxicillin and clavulanate potassium extended-release tablets immediately and promptly report the first signs or symptoms of skin rash, mucosal lesions, or any other sign of hypersensitivity [see Warnings and Precautions (5.2)].
Antibacterial Resistance
Patients should be counseled that antibacterial drugs, including amoxicillin and clavulanate potassium extended-release tablets, should only be used to treat bacterial infections. Antibacterial drugs do not treat viral infections (e.g., the common cold). When amoxicillin and clavulanate potassium extended-release tablets is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may: (1) decrease the effectiveness of the immediate treatment, and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by amoxicillin and clavulanate potassium extended-release tablets or other antibacterial drugs in the future.
Diarrhea
Counsel patients that diarrhea is a common problem caused by antibacterials drugs which usually ends when the antibacterial drug is discontinued. Sometimes after starting treatment with antibacterial drugs, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as 2 or more months after having taken their last dose of the antibacterial drug. If diarrhea is severe or lasts more than 2 or 3 days, patients should contact their physician as soon as possible.
MAALOX® is a registered trademark of Novartis Consumer Health, Inc.
CLINITEST® is a registered trademark of Miles, Inc.
46320723
Manufactured by Sandoz GmbH for Sandoz Inc., Princeton, NJ 08540
- 1000 mg/62.5 mg Label
-
INGREDIENTS AND APPEARANCE
AMOXICILLIN AND CLAVULANATE POTASSIUM
amoxicillin and clavulanate potassium tablet, multilayer, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0781-1943 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMOXICILLIN (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 562.5 mg AMOXICILLIN SODIUM (UNII: 544Y3D6MYH) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 437.5 mg CLAVULANATE POTASSIUM (UNII: Q42OMW3AT8) (CLAVULANIC ACID - UNII:23521W1S24) CLAVULANIC ACID 62.5 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color WHITE (white to cream tinged) Score 2 pieces Shape OVAL Size 22mm Flavor Imprint Code SZ137 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0781-1943-39 40 in 1 BOTTLE; Type 0: Not a Combination Product 04/21/2010 2 NDC:0781-1943-82 28 in 1 BOTTLE; Type 0: Not a Combination Product 04/21/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090227 04/21/2010 Labeler - Sandoz Inc (005387188)