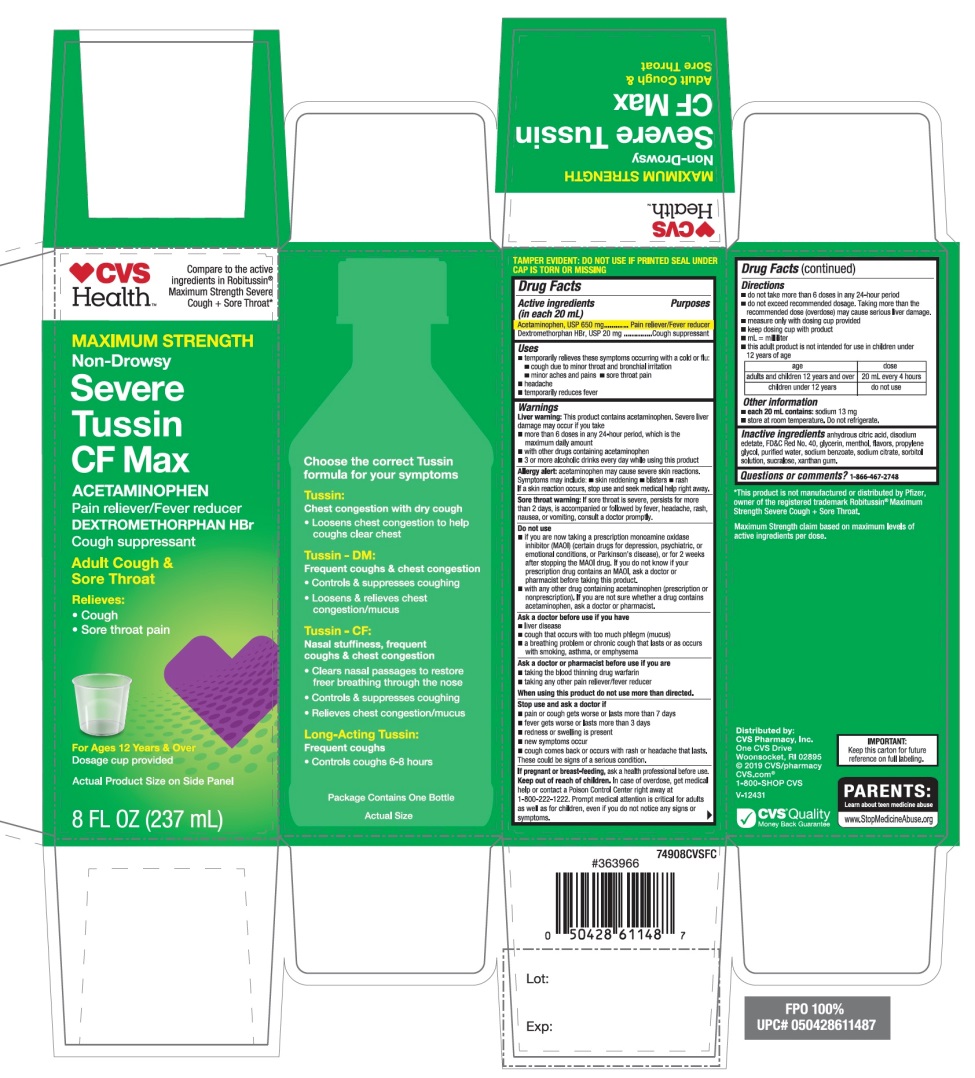
Label: CVS SEVERE TUSSIN CF MAX- acetaminophen, dextromethorphan hbr solution
- NDC Code(s): 69842-947-08
- Packager: CVS PHARMACY
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 23, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each 20 mL)
- Purposes
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- ▪
- more than 6 doses in any 24 hours period, which is the maximum daily amount
- ▪
- with other drugs containing acetaminophen
- ▪
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- ▪
- skin reddening
- ▪
- blisters
- ▪
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
-
Do not use
- ▪
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- ▪
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- When using this product do not use more than directed.
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
Directions
- ▪
- do not take more than 6 doses in any 24-hour period
- ▪
- do not exceed recommended dosage. Taking more than the recommended dose (overdose) may cause serious liver damage.
- ▪
- measure only with dosing cup provided
- ▪
- keep dosing cup with product
- ▪
- mL = milliliter
- ▪
- this adult product is not intended for use in children under 12 years of age
Age
dose
adults and children
12 years and over
20 mL every 4 hours
children under 12 years
do not use
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
Compare to active ingredients in Robitussin® Maximum Strength Severe Cough + Sore Throat*
NDC# 69842-947-08
MAXIMUM STRENGTH
Non–Drowsy
Severe Tussin CF Max
ACETAMINOPHEN
Pain reliever / Fever reducer
DEXTROMETHORPHAN HBr
Cough Suppressant
Adult Cough & Sore Throat
Relieves:
- •
- Cough
- •
- Sore throat pain
For Ages 12 Years & older
Dosage cup provided
Actual Product Size on Side Panel
8 FL OZ (237 mL)
*This product is not manufactured or distributed by Pfizer, owner of the registered trademark Robitussin Maximum Strength Severe Cough + Sore Throat.
Maximum Strength claim based on maximum levels of active ingredients per dose.
IMPORTANT: Keep this carton for future reference on full labeling.
Distributed by:
CVS Pharmacy, Inc.
One CVS Drive
Woonsocket, RI 02895
© 2019 CVS/pharmacy
CVS.com
1-800-SHOP CVS
V-12431
CVS Quality
Money Back Guarantee
-
INGREDIENTS AND APPEARANCE
CVS SEVERE TUSSIN CF MAX
acetaminophen, dextromethorphan hbr solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-947 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg in 20 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color PURPLE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-947-08 1 in 1 CARTON 04/15/2019 1 237 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 04/15/2019 Labeler - CVS PHARMACY (062312574)