Label: NITROGEN gas
- NDC Code(s): 71408-003-01
- Packager: Ineos Pigments ASU LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 25, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Nitrogen
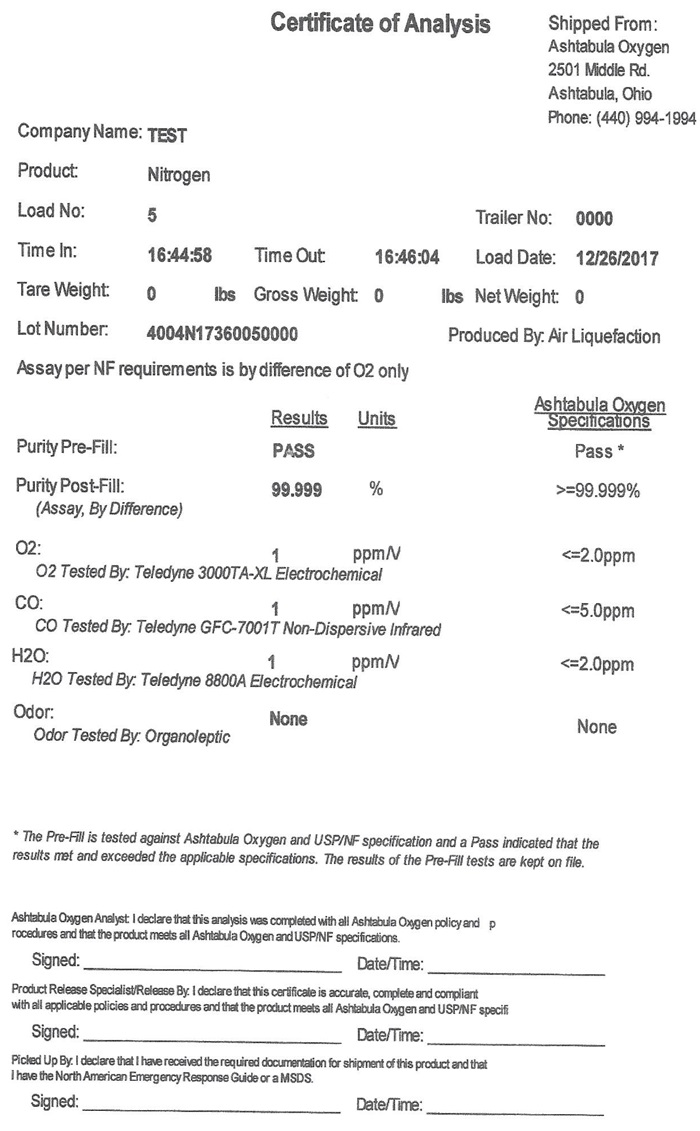
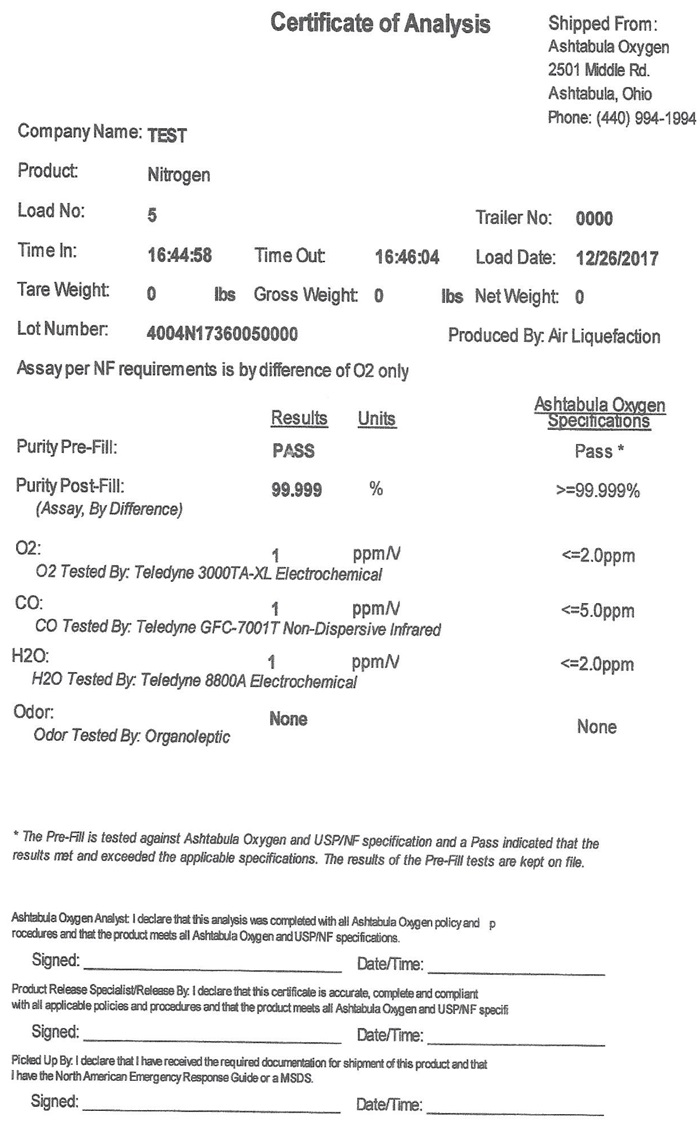
Certificate of Analysis Shipped From:
Ashtabula Oxygen
2501 Middle Rd.
Ashtabula, Ohio
Phone: (440) 994-1994
Company Name: TEST
Product: Nitrogen
Load No: 5 Trailer No: 0000
Time In: 16:44:58 Time Out: 16:46:04 Load Date: 12/26/2017
Tare Weight: 0 Gross Weight: 0 lbs Net Weight: 0 lbs
Lot Number: 4004N17360050000 Produced by: Air Liquefaction
Assay per NF requirements is by difference of O2 only
Ashtabula Oxygen
ResultsUnitsSpecifications
Purity Pre-Fill: PASS Pass*
Purity Post-Fill: 99.99 % >=99.999%
Assay, By Difference)
O2: ppm/V <=2.0ppm
O2 Tested By: Teledyne 3000TA-XL Electrochemcial
CO: ppm/V <=5.0ppm
CO Tested By: Teledyne GFC-7001T Non-Dispersive Infrared
H2O: ppm/V <=2.0ppm
H20 Tested By: Teledyne 8800A Electrochemcial
Odor: None None
Odor Tested By: Organoleptic
- The Pre-Fill is tested against Ashtabula Oxygen and USP/NF specification and a “Pass” indicated that the results met and exceeded the applicable specifications. The results of the Pre-Fill tests are kept on file.
Ashtabula Oxygen Analyst: I declare that this analysis was completed with all Ashtabula Oxygen policy and procedures and that the product meets all Ashtabula Oxygen and USP/NF specifications.
Signed: Date/Time:
Product Release Specialist/Release By: I declare that this certification is accurate, complete and compliant with all applicable policies and procedures and that the product meets all Ashtabula Oxygen and USP/NF specifications.
Signed: Date/Time:
Picked Up By: I declare that I have received the required documentation for shipment of this product and that I have the North American Emergency Response Guide or a MSDS.
Signed: Date/Time:
-
INGREDIENTS AND APPEARANCE
NITROGEN
nitrogen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71408-003 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROGEN (UNII: N762921K75) (NITROGEN - UNII:N762921K75) NITROGEN 99 L in 100 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71408-003-01 10000 L in 1 TANK; Type 0: Not a Combination Product 12/27/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211737 12/27/2017 Labeler - Ineos Pigments ASU LLC (080634966) Registrant - Ineos Pigments ASU LLC (080634966) Establishment Name Address ID/FEI Business Operations Ineos Pigments ASU LLC 080634966 manufacture(71408-003)