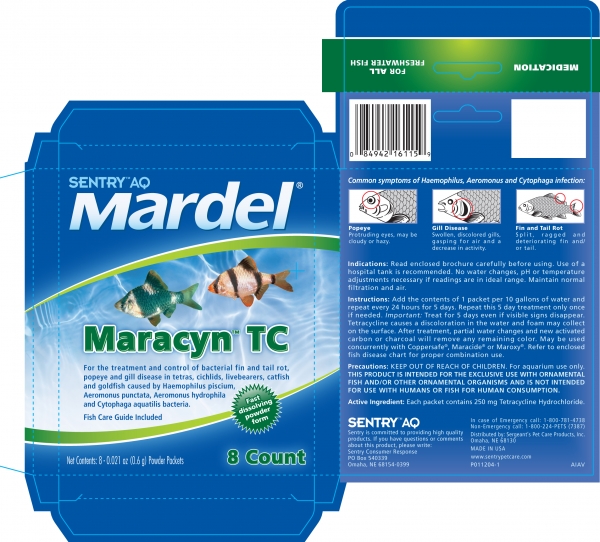
SENTRY AQ MARDEL MARACYN TC- tetracycline hydrochloride powder
Sergeant's Pet Care Products, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Medication for All Freshwater Fish
INDICATIONS AND USAGE
For the treatment and control of bacterial fin and tail rot, popeye and gill disease in tetras, cichlids, livebearers, catfish and goldfish caused by Haemophilus piscium, Aeromonus punctata, Aeromonus hydrophila and cytophaga aquatillis bacteria.
Read enclosed brochure carefully before using. Use of a hospital tank is recommended. No water changes, pH or temperature adjustments necessary if readings are in ideal range. Maintain normal filtration and air.
DOSAGE AND ADMINISTRATION
Add the content of 1 packet per 10 gallons of water and repeat every 24 hours for 5 days. Repeat this 5 day treatment only once, if needed. Important: Treat for 5 days even if visible signs disappear, Tetracycline causes a discoloration in the water and foam may collect on the surface. After treatment, partial water changes and new activated carbon or charcoal will remove any remaining color. May be used concurrently with Coppersafe, Maracide or Maroxy. Refer to enclosed fish disease chart for proper combination use.
| SENTRY AQ MARDEL MARACYN TC
tetracycline hydrochloride powder |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sergeant's Pet Care Products, Inc. (876995171) |