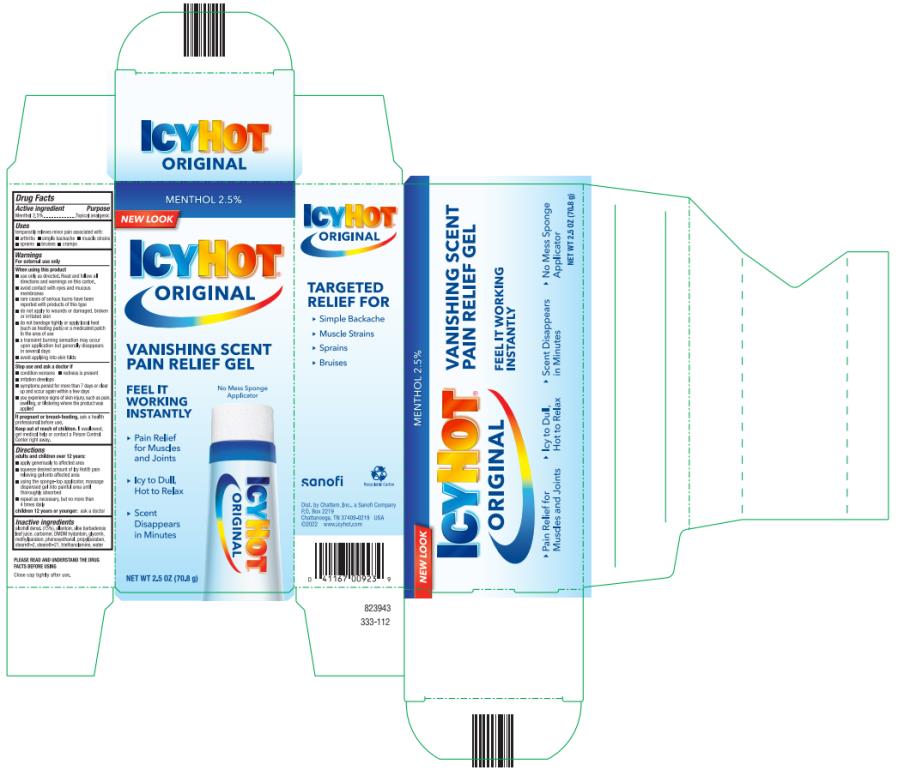
Label: ICY HOT VANISHING SCENT- menthol gel
- NDC Code(s): 41167-0092-0
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 23, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
■ use only as directed. Read and follow all directions and warnings on this carton.
■ avoid contact with eyes and mucous membranes
■ rare cases of serious burns have been reported with products of this type
■ do not apply to wounds or damaged, broken or irritated skin
■ do not bandage tightly or apply local heat (such as heating pads) or a medicated patch to the area of use
■ a transient burning sensation may occur upon application but generally disappears in several days
■ avoid applying into skin folds
-
Directions
adults and children over 12 years:
- apply generously to affected area
- squeeze desired amount of Icy Hot® pain relieving gel onto affected area
- using the sponge-top applicator, massage dispensed gel into painful area until thoroughly absorbed
- repeat as necessary, but not more than 4 times daily
children 12 years or younger: ask a doctor
- apply generously to affected area
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ICY HOT VANISHING SCENT
menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0092 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.025 g in 1 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) ALLANTOIN (UNII: 344S277G0Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) DMDM HYDANTOIN (UNII: BYR0546TOW) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLPARABEN (UNII: Z8IX2SC1OH) STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0092-0 1 in 1 CARTON 03/01/2007 1 71 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/01/2007 Labeler - Chattem, Inc. (003336013) Establishment Name Address ID/FEI Business Operations CHATTEM, INC. 830410721 manufacture(41167-0092) Establishment Name Address ID/FEI Business Operations CHATTEM, INC. 003336013 manufacture(41167-0092)