Label: REFRESH NATURE CREAM- citric acid, glycerin cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 82790-301-01 - Packager: GP International Corp.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 22, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
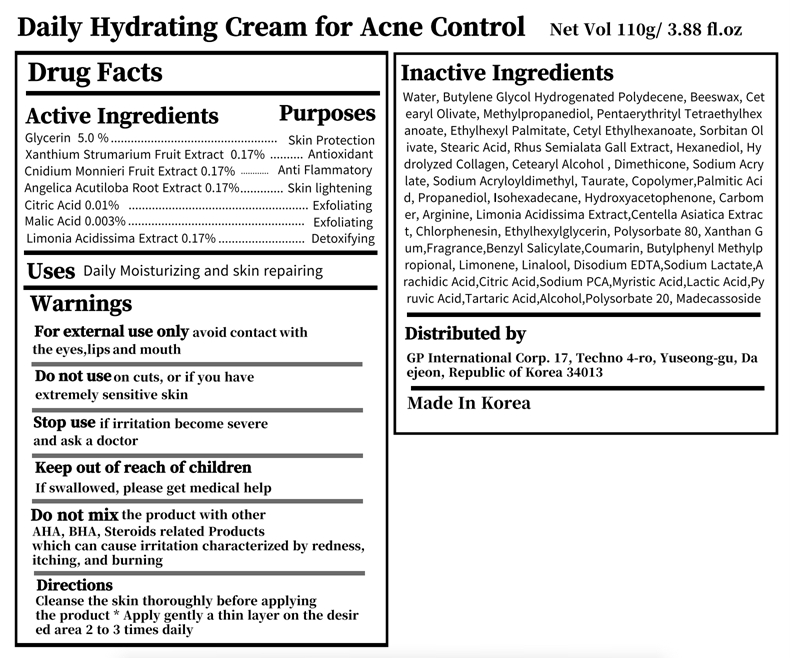
- purpose
- Use
- Warnings
- Warnings
- Warnings
- Warnings
- Warnings
- Directions
- active ingredient
-
Inactive Ingredients
Water, Butylene Glycol Hydrogenated Polydecene, Beeswax, Cetearyl Olivate, Methylpropanediol, Pentaerythrityl Tetraethylhexanoate, Ethylhexyl Palmitate, Cetyl Ethylhexanoate, Sorbitan Olivate, Stearic Acid, Rhus Semialata Gall Extract, Hexanediol, Hydrolyzed Collagen, Cetearyl Alcohol , Dimethicone, Sodium Acrylate, Sodium Acryloyldimethyl, Taurate, Copolymer,Palmitic Acid, Propanediol, Isohexadecane, Hydroxyacetophenone, Carbomer, Arginine, Limonia Acidissima Extract,Centella Asiatica Extract, Chlorphenesin, Ethylhexylglycerin, Polysorbate 80, Xanthan Gum,Fragrance,Benzyl Salicylate,Coumarin, Butylphenyl Methylpropional, Limonene, Linalool, Disodium EDTA,Sodium Lactate,Arachidic Acid,Citric Acid,Sodium PCA,Myristic Acid,Lactic Acid,Pyruvic Acid,Tartaric Acid,Alcohol,Polysorbate 20, Madecassoside
- Daily Hydrating Cream for Acne Control
-
INGREDIENTS AND APPEARANCE
REFRESH NATURE CREAM
citric acid, glycerin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82790-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength XANTHIUM STRUMARIUM FRUIT (UNII: TN770YC17C) (XANTHIUM STRUMARIUM FRUIT - UNII:TN770YC17C) XANTHIUM STRUMARIUM FRUIT 0.17 g in 100 g CNIDIUM MONNIERI FRUIT (UNII: V1IA3S3CUS) (CNIDIUM MONNIERI FRUIT - UNII:V1IA3S3CUS) CNIDIUM MONNIERI FRUIT 0.17 g in 100 g MALIC ACID (UNII: 817L1N4CKP) (MALIC ACID - UNII:817L1N4CKP) MALIC ACID 0.003 g in 100 g GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 5 g in 100 g ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) (ANGELICA ACUTILOBA ROOT - UNII:3W51R3EK30) ANGELICA ACUTILOBA ROOT 0.17 g in 100 g LIMONIA ACIDISSIMA WHOLE (UNII: MU5C4X2819) (LIMONIA ACIDISSIMA WHOLE - UNII:MU5C4X2819) LIMONIA ACIDISSIMA WHOLE 0.17 g in 100 g CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 0.01 g in 100 g Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM ACRYLOYLDIMETHYLTAURATE (UNII: 2T9Q6EKI0G) TAURINE (UNII: 1EQV5MLY3D) GLATIRAMER ACETATE (UNII: 5M691HL4BO) POLYSORBATE 20 (UNII: 7T1F30V5YH) MADECASSOSIDE (UNII: CQ2F5O6YIY) MARINE COLLAGEN, SOLUBLE (UNII: 8JC99XGU4W) SODIUM ACRYLATE (UNII: 7C98FKB43H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) HYDROGENATED POLYDECENE TYPE I (UNII: U333RI6EB7) LACTIC ACID (UNII: 33X04XA5AT) ISOHEXADECANE (UNII: 918X1OUF1E) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) ARACHIDIC ACID (UNII: PQB8MJD4RB) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) MYRISTIC ACID (UNII: 0I3V7S25AW) PALMITIC ACID (UNII: 2V16EO95H1) YELLOW WAX (UNII: 2ZA36H0S2V) CETEARYL OLIVATE (UNII: 58B69Q84JO) METHYLPROPANEDIOL (UNII: N8F53B3R4R) PENTAERYTHRITYL TETRAETHYLHEXANOATE (UNII: XJ7052W897) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) XANTHAN GUM (UNII: TTV12P4NEE) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) RHUS CHINENSIS GALL (UNII: 4W3Y2V7J3R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) BENZYL SALICYLATE (UNII: WAO5MNK9TU) COUMARIN (UNII: A4VZ22K1WT) LIMONENE, (+/-)- (UNII: 9MC3I34447) PROPANEDIOL (UNII: 5965N8W85T) ARGININE (UNII: 94ZLA3W45F) CENTELLA ASIATICA TRITERPENOIDS (UNII: 4YS74Q4G4J) POLYSORBATE 80 (UNII: 6OZP39ZG8H) LINALOOL, (+/-)- (UNII: D81QY6I88E) SORBITAN OLIVATE (UNII: MDL271E3GR) HEXANEDIOL (UNII: ZIA319275I) DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) ETHYLHEXYL PALMITATE (UNII: 2865993309) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) FRAGRANCE LEMON ORC2001060 (UNII: K1725A7G95) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) SODIUM LACTATE (UNII: TU7HW0W0QT) PYRUVIC ACID (UNII: 8558G7RUTR) TARTARIC ACID (UNII: W4888I119H) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82790-301-01 110 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/22/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 06/22/2022 Labeler - GP International Corp. (695896955) Registrant - GP International Corp. (695896955) Establishment Name Address ID/FEI Business Operations GP International Corp. 695896955 manufacture(82790-301)