MOTION SICKNESS- dimenhydrinate tablet
GREAT LAKES WHOLESALE, MARKETING, & SALES, INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Healthcare 44-198
Uses
for prevention and treatment of these symptoms associated with motion sickness:
- nausea
- vomiting
- dizziness
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
Directions
- to prevent motion sickness, the first dose should be taken one-half to one hour before starting activity
| adults and children 12 years and over | 1 to 2 tablets every 4-6 hours; do not exceed 8 tablets in 24 hours, or as directed by a doctor |
| children 6 to under 12 years | ½ to 1 tablet every 6-8 hours; do not exceed 3 tablets in 24 hours, or as directed by a doctor |
| children 2 to under 6 years | ½ tablet every 6-8 hours; do not exceed 1½ tablets in 24 hours, or as directed by a doctor |
Other information
-
each tablet contains: calcium 35 mg
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º- 86ºF)
- protect from moisture
- see end flap for expiration date and lot number
Inactive ingredients
croscarmellose sodium, dibasic calcium phosphate dihydrate, magnesium stearate, microcrystalline cellulose, silicon dioxide, stearic acid
Principal Display Panel
HEALTHCARE™
NDC 64092-600-12
*Compare to the active ingredient
in Dramamine® Original Formula
Motion Sickness
Dimenhydrinate, 50 mg Each
Antiemetic
Prevents:
• Nausea • Dizziness • Vomiting
From Motion Sickness
12 TABLETS
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS
OPENED OR IF BLISTER UNIT IS TORN, BROKEN
OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by Medtech Products Inc.,
owner of the registered trademark Dramamine® Original Formula.
50844 REV0518G19802
Distributed by:
Great Lakes Wholesale & Marketing L.L.C.
3729 Patterson Ave., S.E.
Grand Rapids, MI 49512 www.glwholesale.com
HEALTHCARE GUARANTEE
If you are not completely satisfied with this
product, regardless of reason, return your unused
portion to Great Lakes Wholesale for a full refund
Healthcare 44-198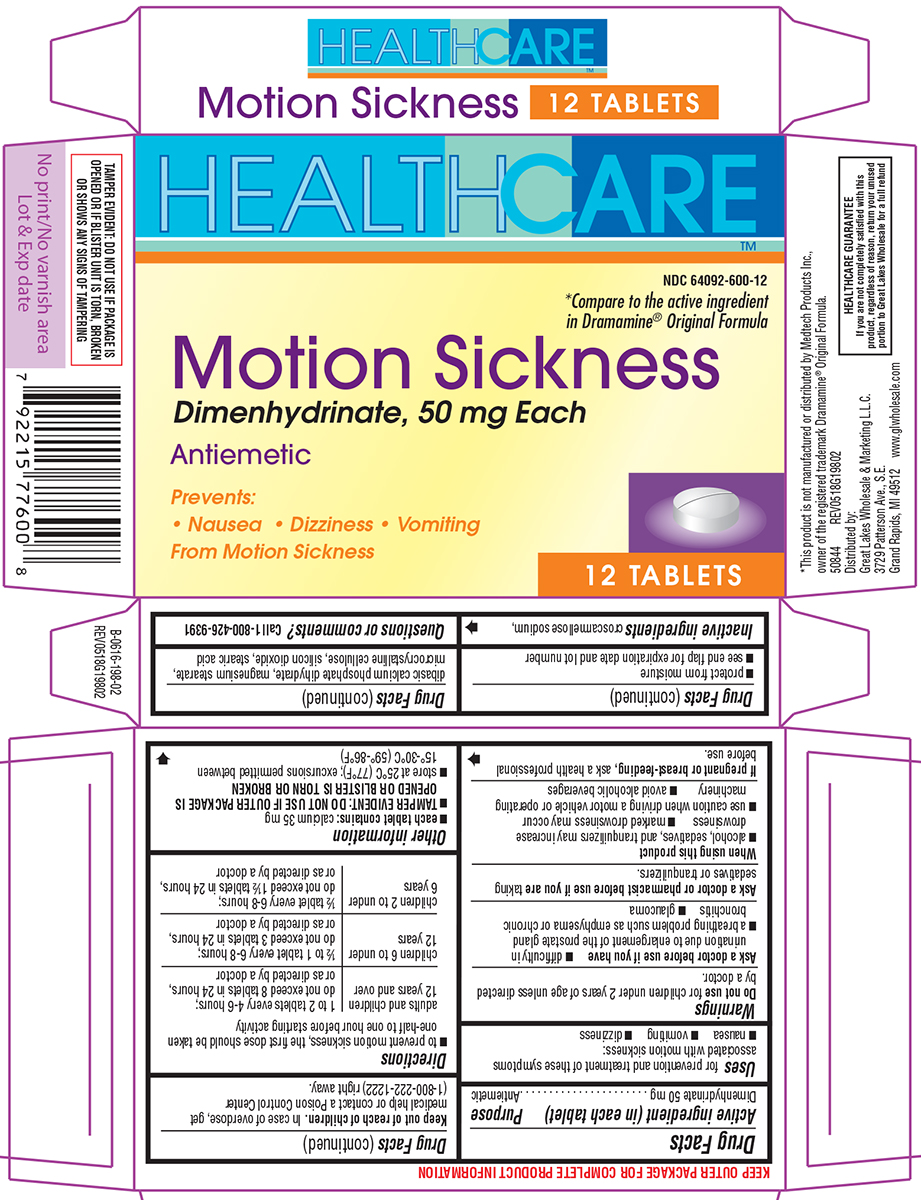
| MOTION SICKNESS
dimenhydrinate tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - GREAT LAKES WHOLESALE, MARKETING, & SALES, INC. (361925498) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(64092-600) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | pack(64092-600) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(64092-600) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | pack(64092-600) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(64092-600) | |