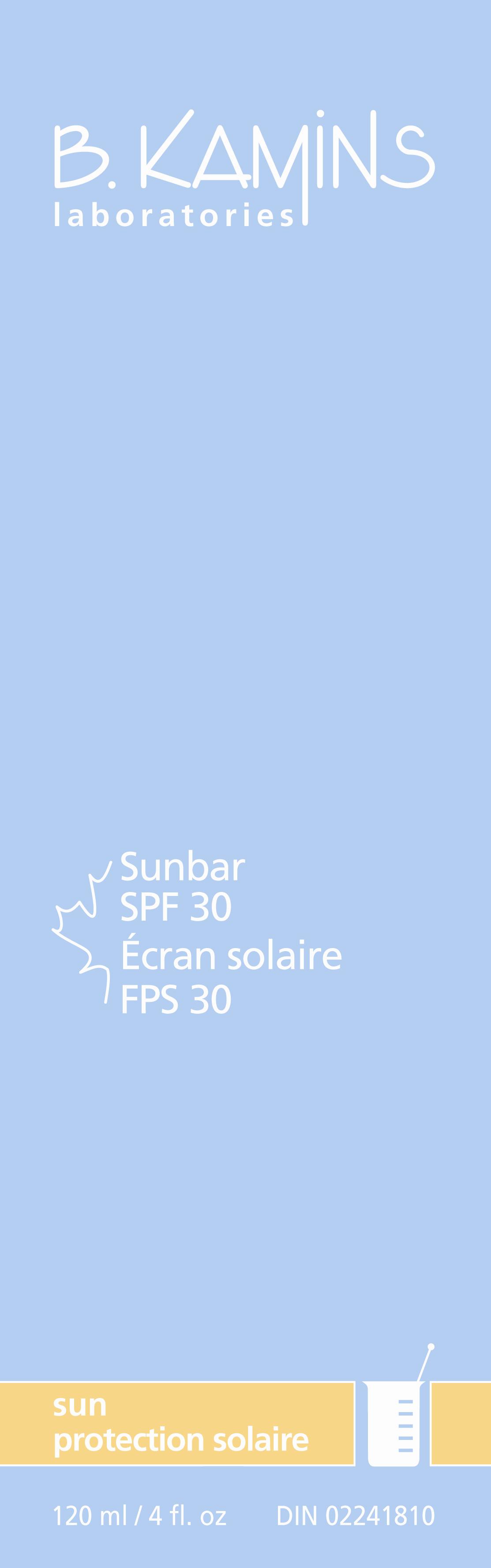
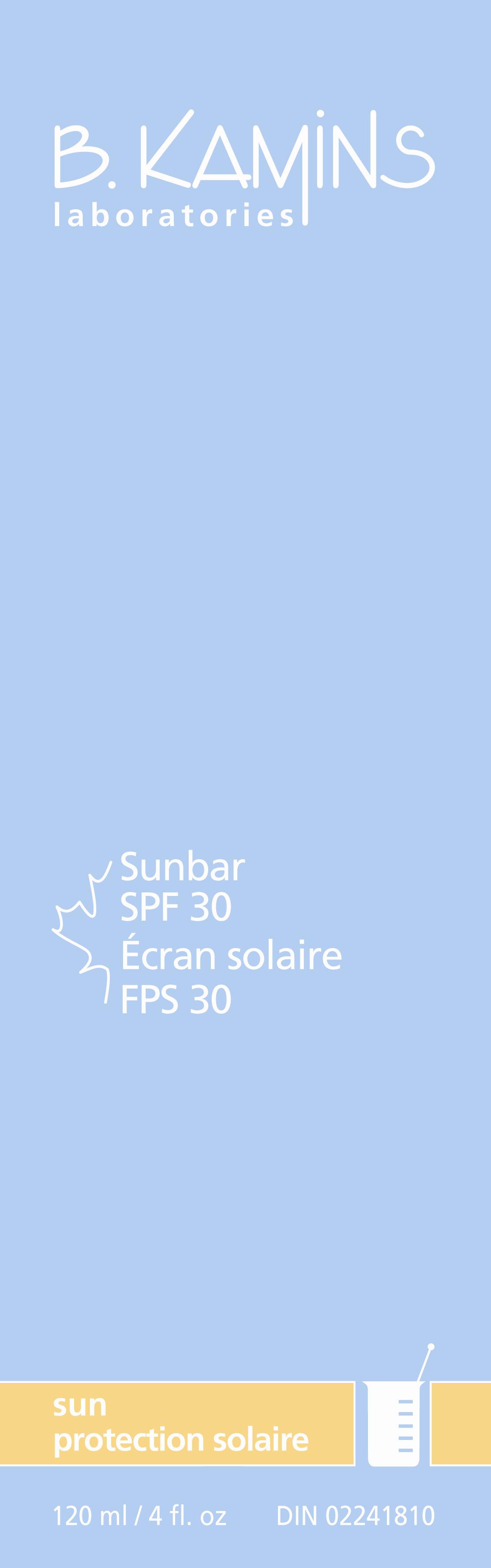
Label: SUNBAR SPF 30- avobenzone octinoxate oxybenzone titanium dioxide lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 63550-811-10 - Packager: Kamins Dermatologics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 15, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
This oil and fragrance-free broad spectrum UVA/UVB sunscreen provides important protection against the sun’s harmful rays. Recommended for use on face and body, for everyday wear and outdoor activities. This cosmetically elegant formula is non-greasy, leaves a matte finish, and can be reapplied over makeup.
• fragrance-free • oil-free
• for face and body
Uses
■ Helps prevent sunburn.■ Higher SPF gives more sunburn protection.
■ Provides high protection against sunburn.
-
WARNINGS
Warnings
For external use only.
When using this product, keep out of eyes. If contact occurs, rinse abundantly with water to remove.
Stop use and ask a doctor if rash or irritation develops and lasts.
If swallowed, seek medical assistance or contact a Poison Control Center immediately.
Other information
Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risks of skin aging, skin cancer, and other harmful effects of the sun.Store at room temperature 15-30°C (59-86°F).
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SUNBAR SPF 30
avobenzone octinoxate oxybenzone titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63550-811 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 25 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 6 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ACER SACCHARUM SAP (UNII: 75UOH57984) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) CETYL ALCOHOL (UNII: 936JST6JCN) STEARIC ACID (UNII: 4ELV7Z65AP) DIETHANOLAMINE CETYL PHOSPHATE (UNII: 4UG0316V9S) PANTHENOL (UNII: WV9CM0O67Z) DIMETHICONE (UNII: 92RU3N3Y1O) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63550-811-10 120 mL in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 10/15/2010 Labeler - Kamins Dermatologics Inc. (254050784) Registrant - Kamins Dermatologics Inc. (254050784) Establishment Name Address ID/FEI Business Operations Kamins Dermatologics Inc. 254050784 manufacture, pack, label