BISMATROL- bismuth subsalicylate tablet, chewable
Preferred Pharmaceuticals Inc.
----------
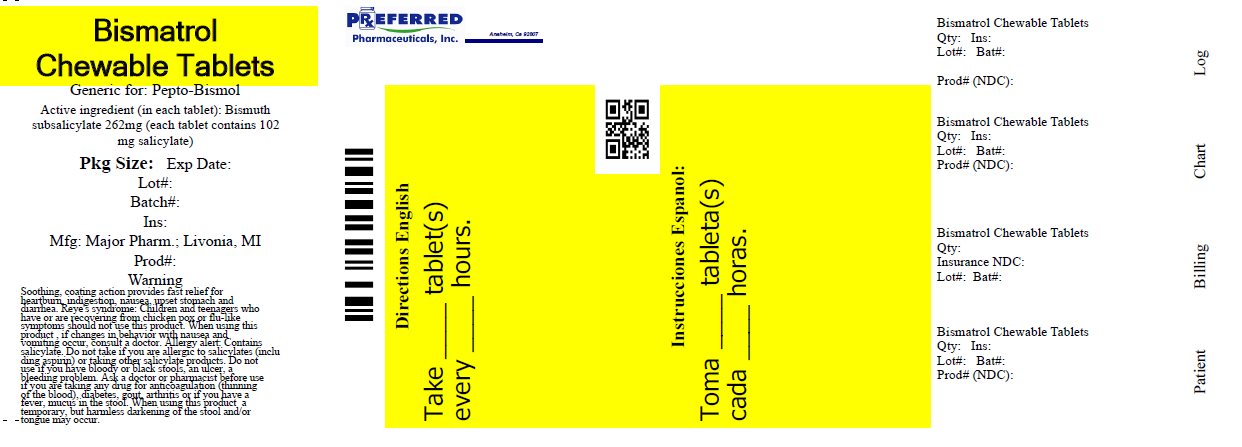
Major Bismatrol Chewable Tablets
Active ingredient (in each tablet)
Bismuth subsalicylate 262 mg
(total salicylate 102 mg per tablet)
Uses
relieves:
- •
- diarrhea
- •
- heartburn
- •
- indigestion
- •
- nausea
- •
- upset stomach associated with these symptoms
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. when using this product , if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are
- •
- allergic to salicylates (including aspirin)
- •
- taking other salicylate products
Ask a doctor or pharmacist before use if you are taking any drug for
- •
- anticoagulation (thinning of the blood)
- •
- diabetes
- •
- gout
- •
- arthritis
Keep Out of Reach of Children
Keep Out of Reach of Children - In case of overdose, get medical help or contact a Poison Control Center immediately
Directions
- •
- chew or dissolve in mouth
- •
- adults and children 12 years and over: 2 tablets every 1/2 to 1 hour as needed
- •
- do not take more than 8 doses (16 tablets) in 24 hours
- •
- children under 12 years: ask a doctor
- •
- drink plenty of fluids to help prevent dehydration caused by diarrhea.
Other information:
each tablet contains:
- •
- sodium less than 1 mg
- •
- salicylate 102 mg
- •
- very low sodium
- •
- avoid excessive heat (over 104˚F or 40˚C)
- •
- TAMPER EVIDENT:Do not use if individual compartments are torn or missing.
| BISMATROL
bismuth subsalicylate tablet, chewable |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Preferred Pharmaceuticals Inc. (791119022) |
| Registrant - Preferred Pharmaceuticals Inc. (791119022) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Preferred Pharmaceuticals Inc. | 791119022 | RELABEL(68788-7140) | |
Revised: 3/2024
Document Id: 49e3c9c1-9bb3-4d9b-8bc3-08b64d73df6a
Set id: e1d5e365-5dc8-4d77-a730-f59eeb8fb01a
Version: 7
Effective Time: 20240311
Preferred Pharmaceuticals Inc.