POTASSIUM IODIDE- potassium iodide solution
Laser Pharmaceuticals Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Potassium Iodide Oral Solution, USP (Saturated)
1 g/mL
Rx only
Description
Potassium Iodide Oral Solution, USP is a saturated solution of potassium iodide containing 1 gram of potassium iodide per mL.
Clinical Pharmacology
Potassium iodide is thought to act as an expectorant by increasing respiratory tract secretions and thereby decreasing the viscosity of mucus.
Indications and Usage
Potassium Iodide Oral Solution, USP is for use as an expectorant in the symptomatic treatment of chronic pulmonary diseases where tenacious mucus complicates the problem, including bronchial asthma, bronchitis and pulmonary emphysema.
Warnings
Potassium iodide can cause fetal harm, abnormal thyroid function, and goiter when administered to a pregnant woman. Because of the possible development of fetal goiter, if the drug is used during pregnancy or if the patient becomes pregnant during therapy, apprise the patient of the potential hazard.
Precautions
General
In some patients, prolonged use of iodides can lead to hypothyroidism. Iodides should be used with caution in patients having Addison's disease, cardiac disease, hyperthyroidism, myotonia congenita, tuberculosis, acute bronchitis, or renal function impairment.
Drug Interactions
Concurrent use with lithium or antithyroid drugs may potentiate the hypothyroid and goitrogenic effects of these medications. Concurrent use with potassium-containing medications, potassium-sparing diuretics and angiotensin-converting enzyme inhibitors (ACE inhibitors) may result in hyperkalemia and cardiac arrhythmias or cardiac arrest.
Adverse Reactions
The most frequent adverse reactions to potassium iodide are stomach upset, diarrhea, nausea, vomiting, stomach pain, skin rash, and salivary gland swelling or tenderness. Less frequent adverse reactions include gastrointestinal bleeding, confusion, irregular heartbeat, numbness, tingling, pain or weakness in hands or feet, unusual tiredness, weakness or heaviness of legs, fever, and swelling of neck or throat. Thyroid adenoma, goiter, and myxedema are possible side effects.
Iodism or chronic iodine poisoning may occur during prolonged treatment or with the use of high doses. The symptoms of iodism include burning of mouth or throat, severe headache, metallic taste, soreness of teeth and gums, symptoms of head cold, irritation of the eyes with swelling of the eyelids, unusual increase in salivation, acneform skin lesions in the seborrheic areas, and rarely, severe skin eruptions. If symptoms of iodism appear, the drug should be withdrawn and the patient given appropriate supportive therapy.
Hypersensitivity to iodides may occur and may be manifested by angioedema, cutaneous and mucosal hemorrhage, and signs and symptoms resembling serum sickness, such as fever, arthralgia, lymph node enlargement, and eosinophilia.
Overdosage
Acute toxicity from potassium iodide is relatively rare. An occasional individual may show marked sensitivity and the onset of acute poisoning can occur immediately or hours after administration. Angioedema, laryngeal edema and cutaneous hemorrhages may occur.
Iodism or chronic iodine poisoning may occur during prolonged treatment or with the use of high doses. Symptoms of iodism typically disappear soon after discontinuation of the drug. Abundant fluid and salt intake aids in iodide elimination.
Dosage and Administration
Adults - 0.3 mL (300 mg) or 0.6 mL (600 mg) diluted in one glassful of water, fruit juice or milk 3 to 4 times daily. To minimize gastric irritation, take with food or milk.
This medication should be used no longer than necessary to produce the desired effect.
How Supplied
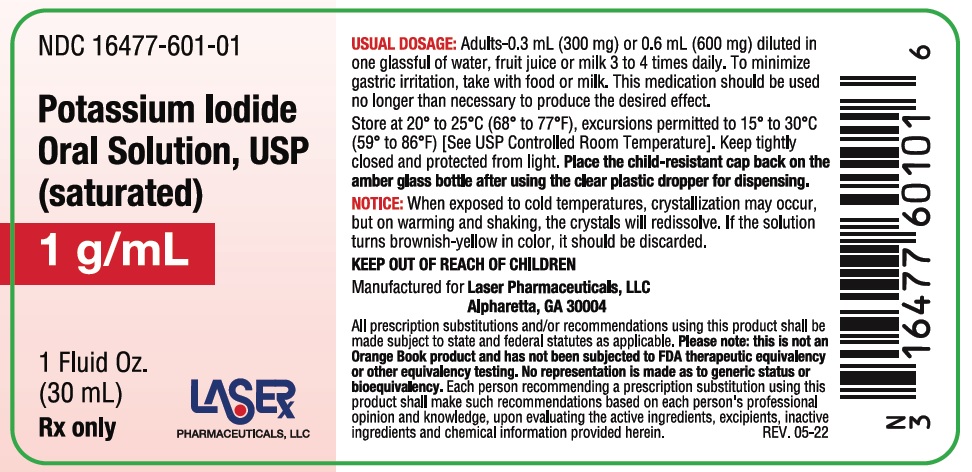
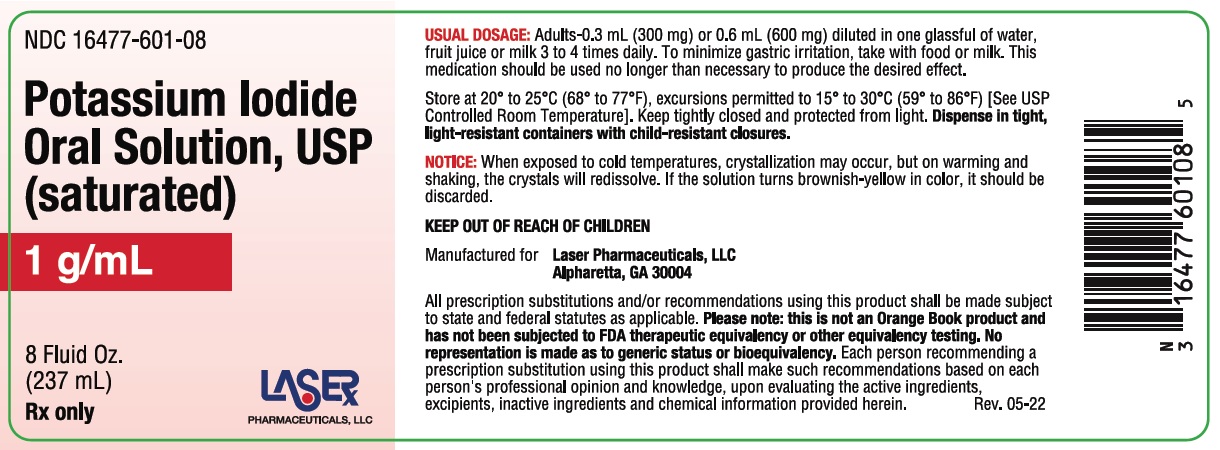
Potassium Iodide Oral Solution, USP is supplied in 1 fluid ounce (30 mL) bottles (NDC 16477-601-01) with a calibrated dropper marked to deliver 0.3 mL (300 mg) and 0.6 mL (600 mg); and 8 fluid ounce (237 mL) bottles (NDC 16477-601-08). Inactive ingredient: Sodium thiosulfate as a preservative.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Keep tightly closed and protected from light.
For the 237mL bottle, dispense in tight, light-resistant containers with child-resistant closures. For the 30mL bottle, place the child-resistant cap back on the amber glass bottle after using the clear plastic dropper for dispensing.
Notice: When exposed to cold temperatures, crystallization may occur, but on warming and shaking, the crystals will redissolve. If the solution turns brownish-yellow in color, it should be discarded.
Potassium Iodide Oral Solution, USP (saturated)
1g/mL
1 Fluid Oz. (30 mL)
8 Fluid Oz. (237 mL)
Rx only
LASER Pharmaceuticals, LLC
USUAL DOSAGE: Adults - 0.3 mL (300 mg) or 0.6 mL (600 mg) diluted in one glassful of water, fruit juice or milk 3 to 4 times daily. To minimize gastric irritation, take with food or milk. This medication should be used no longer than necessary to produced the desired affect.
Store at 20° to 25°C (68° to 77°F), excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Keep tightly closed and protected from light. Dispense in tight, light-resistant containers with child-resitant closures.
Notice: When exposed to cold temperatures, crystallization may occur, but on warming and shaking, the crystals will redissolve. If the solution turns brownish-yellow in color, it should be discarded.
KEEP OUT OF REACH OF CHILDREN
Manufactured for Laser Pharmaceuticals, LLC, Alpharetta, GA 30004
All prescription substitutions and/or recommendations using this produvt shall be made subject to state and federal statutes as applicable. Please note: this is not an Orange Book product and has not been subjected to FDA therapeutic equivalency or other equivalency testing. No representation is made as to generic status or bioequivalency. Each person recommending a prescription substitution using this product shall make such recommendations based on each person's professional opinion and knowledge, upon evaluating the active ingredients, excipients, inactive ingredients and chemical informaiton provided herein. Rev. 05-22
| POTASSIUM IODIDE
potassium iodide solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Laser Pharmaceuticals Inc. (614417132) |