TEMODAL- temozolomide capsule
TEMOZOLOMIDE- temozolomide capsule
Orion Corporation, Orion Pharma
----------
TEMODAL
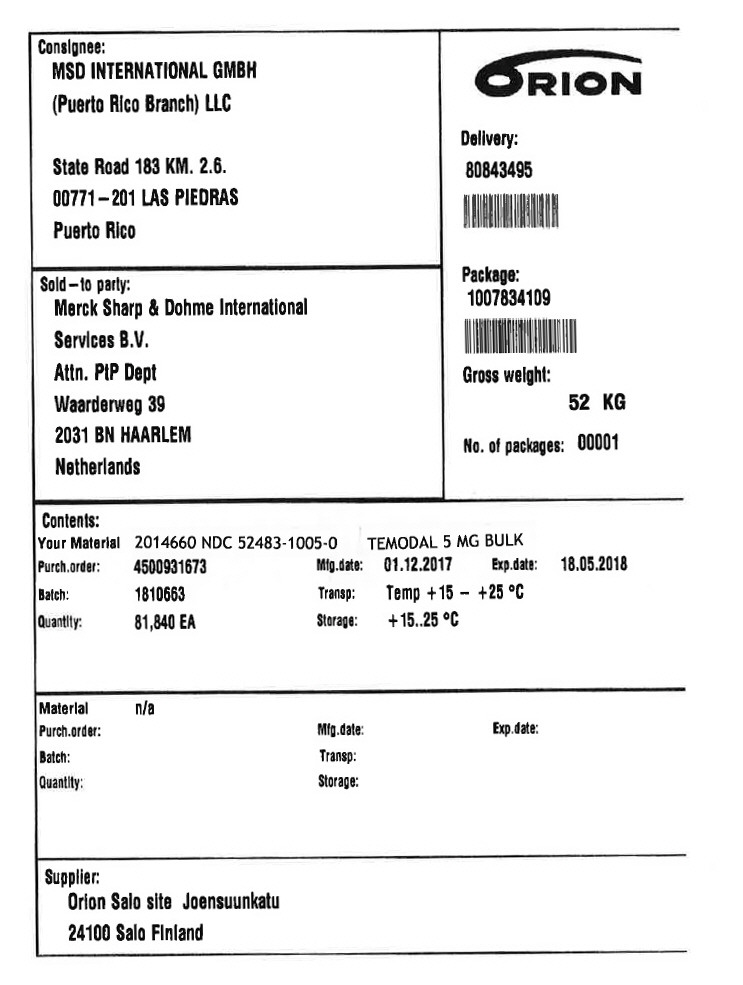
PRINCIPAL DISPLAY PANEL - Shipping Label - 5 MG
Consignee:
MSD INTERNATIONAL GMBH
(Puerto Rico Branch) LLC
State Road 183 KM. 2.6.
00771–201 LAS PIEDRAS
Puerto Rico
Sold–to party:
Merck Sharp & Dohme lnternational
Services B.V.
Attn. PtP Dept
Waarderweg 39
2031 BN HAARLEM
Netherlands
ORION
Delivery:
80843495
Package:
1007834109
Gross weight:
52 KG
No. of packages: 00001
Contents:
Your Material 2014660
NDC 52483-1005-0
TEMODAL 5 MG BULK
Purch.order: 4500931673
Batch: 1810663
Quantity: 81,840 EA
Mfg.date: 01.12.2017
Transp: Temp +15 – +25 °C
Storage: +15..25 °C
Exp.date: 18.05.2018
Material n/a
Purch.order:
Batch:
Quantity:
Mfg.date:
Transp:
Storage:
Exp.date:
Supplier:
Orion Salo site Joensuunkatu
24100 Salo Finland
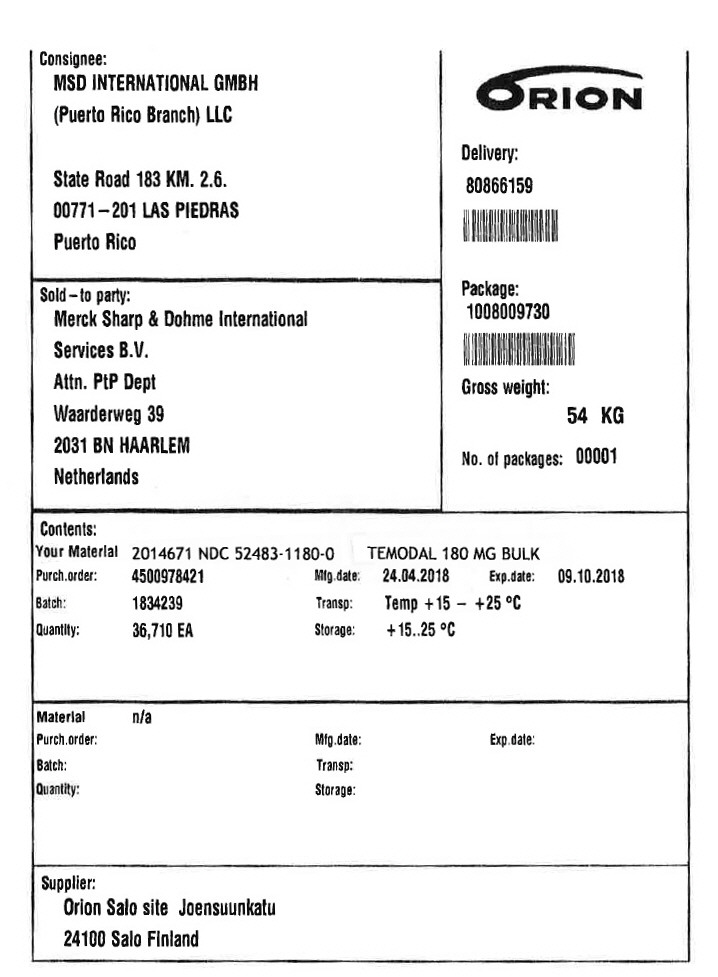
PRINCIPAL DISPLAY PANEL - Shipping Label - 180 MG
Consignee:
MSD INTERNATIONAL GMBH
(Puerto Rico Branch) LLC
State Road 183 KM. 2.6.
00771–201 LAS PIEDRAS
Puerto Rico
Sold–to party:
Merck Sharp & Dohme lnternational
Services B.V.
Attn. PtP Dept
Waarderweg 39
2031 BN HAARLEM
Netherlands
ORION
Delivery:
80866159
Package:
1008009730
Gross weight:
54 KG
No. of packages: 00001
Contents:
Your Material 2014671
NDC 52483-1180-0
TEMODAL 180 MG BULK
Purch.order: 4500978421
Batch: 1834239
Quantity: 36,710 EA
Mfg.date: 24.04.2018
Transp: Temp +15 – +25 °C
Storage: +15..25 °C
Exp.date: 09.10.2018
Material n/a
Purch.order:
Batch:
Quantity:
Mfg.date:
Transp:
Storage:
Exp.date:
Supplier:
Orion Salo site Joensuunkatu
24100 Salo Finland
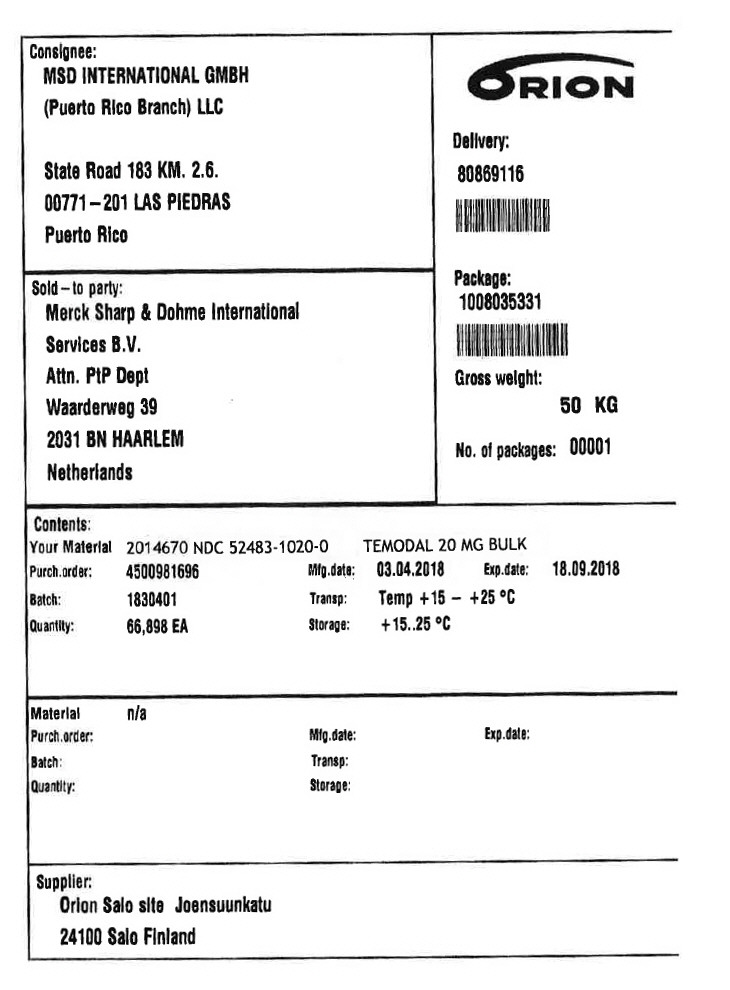
PRINCIPAL DISPLAY PANEL - Shipping Label - 20 MG
Consignee:
MSD INTERNATIONAL GMBH
(Puerto Rico Branch) LLC
State Road 183 KM. 2.6.
00771–201 LAS PIEDRAS
Puerto Rico
Sold–to party:
Merck Sharp & Dohme lnternational
Services B.V.
Attn. PtP Dept
Waarderweg 39
2031 BN HAARLEM
Netherlands
ORION
Delivery:
80869116
Package:
1008035331
Gross weight:
50 KG
No. of packages: 00001
Contents:
Your Material 2014670
NDC 52483-1020-0
TEMODAL 20 MG BULK
Purch.order: 4500981696
Batch: 1830401
Quantity: 66,898 EA
Mfg.date: 03.04.2018
Transp: Temp +15 – +25 °C
Storage: +15..25 °C
Exp.date: 18.09.2018
Material n/a
Purch.order:
Batch:
Quantity:
Mfg.date:
Transp:
Storage:
Exp.date:
Supplier:
Orion Salo site Joensuunkatu
24100 Salo Finland
PRINCIPAL DISPLAY PANEL - Shipping Label - 250 MG
Consignee:
MSD INTERNATIONAL GMBH
(Puerto Rico Branch) LLC
State Road 183 KM. 2.6.
LAS PIEDRAS
00771–2011 Puerto Rico
Puerto Rico
Sold–to party:
Merck Sharp & Dohme International
Services B.V.
Attn. PtP Dept
Waarderweg 39
2031 BN HAARLEM
Netherlands
ORION
Delivery:
80866160
Package:
1008009939
Gross weight:
41 KG
No. of packages: 00001
Contents:
Your Material 2015650 NDC 52483-1250-0
TEMODAL 250 MG BULK
Purch.order: 4500978424
Batch: 1834343
Quantity: 25,205 EA
Mfg.date: 24.04.2018
Transp: Temp +15 – +25 °C
Storage: +15..25 °C
Exp.date: 09.10.2018
Material n/a
Purch.order:
Batch:
Quantity:
Mfg.date:
Transp:
Storage:
Exp.date
Supplier:
Orion Salo site Joensuunkatu
24100 Salo Finland

| TEMODAL
temozolomide capsule |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| TEMOZOLOMIDE
temozolomide capsule |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| TEMOZOLOMIDE
temozolomide capsule |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| TEMOZOLOMIDE
temozolomide capsule |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Orion Corporation, Orion Pharma (539763727) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Orion Corporation, Orion Pharma | 537940319 | MANUFACTURE(52483-1005, 52483-1020, 52483-1180, 52483-1250) , ANALYSIS(52483-1005, 52483-1020, 52483-1180, 52483-1250) | |