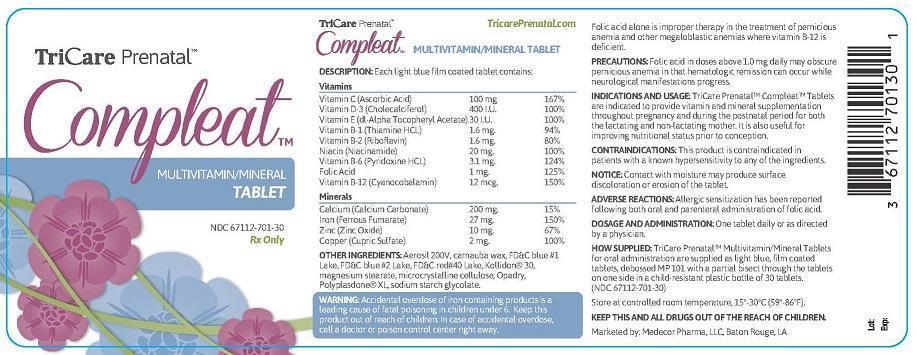
DESCRIPTION: Each light blue film coated tablet contains:
Vitamins
- Vitamin C (Ascorbic Acid) 100 mg 167%
- Vitamin D-3 (Cholecalciferol) 400 I.U. 100%
- Vitamin E (dl-Alpha Tocopheryl Acetate) 30 I.U. 100%
- Vitamin B-1 (Thiamine HCL) 1.6 mg. 94%
- Vitamin B-2 (Riboflavin) 1.6 mg. 80%
- Niacin (Niacinamide) 20 mg. 100%
- Vitamin B-6 (Pyridoxine HCL) 3.1 mg. 124%
- Folic Acid 1 mg. 125%
- Vitamin B-12 (Cyanocobalamin) 12 mcg. 150%
Minerals
- Calcium (Calcium Carbonate) 200 mg. 15%
- Iron (Ferrous Fumarate) 27 mg. 150%
- Zinc (Zinc Oxide) 10 mg. 67%
- Copper (Cupric Sulfate) 2 mg. 100%
DESCRIPTION: Each pink chewable softgel contains:
Essential Fatty Acids
Fish Oil Concentrate
- DHA (Docosahexaenoic acid) 135.0 mg
- EPA (Eicosapentaenoic acid) 33.80 mg
- Other Omega-3’s 71.20 mg
DESCRIPTION: Each light blue film coated tablet contains:
OTHER INGREDIENTS: Aerosil 200V, carnauba wax, FD&C blue #1 Lake, FD&C blue #2 Lake, FD&C red#40 Lake, Kollidon® 30,
magnesium stearate, microcrystalline cellulose, Opadry, Polyplasdone® XL, sodium starch glycolate.
DESCRIPTION: Each pink chewable softgel contains:
OTHER INGREDIENTS: Highly refined and concentrated Omega-3 Fish Oil (Tuna) capsule (gelatin, potato starch, cochineal C175470,
iron oxide black C177499, titanium dioxide), coconut oil-fractionated, lecithin, natural vitamin E (d-aplha -Tocopherol
3.22 mg as vitamin E preparation USP), TS- 12550/ ARTG 13165, sucralose, xylitol (this material contains galactose, glucose,
sucrose, invert sugar, mannitol and sorbitol) citric acid-anhydrous, orange oil, forest fruits flavour 174289941/ARTG-11546.