OXYCODONE AND ACETAMINOPHEN- oxycodone hydrochloride and acetaminophen tablet
Bryant Ranch Prepack
----------
Oxycodone and Acetaminophen Tablets USP 7.5 mg*/500 mg
DESCRIPTION
Each tablet, for oral administration, contains oxycodone hydrochloride and acetaminophen in the following strengths:
- Oxycodone Hydrochloride USP ..........................................................................5 mg*
- Acetaminophen USP ........................................................................................325 mg
- *5 mg oxycodone HCl is equivalent to 4.4815 mg of oxycodone.
- Oxycodone Hydrochloride USP .......................................................................7.5 mg*
- Acetaminophen USP ........................................................................................325 mg
- *7.5 mg oxycodone HCl is equivalent to 6.7228 mg of oxycodone.
- Oxycodone Hydrochloride USP .......................................................................7.5 mg*
- Acetaminophen USP ........................................................................................500 mg
- *7.5 mg oxycodone HCl is equivalent to 6.7228 mg of oxycodone.
- Oxycodone Hydrochloride USP .........................................................................10 mg*
- Acetaminophen USP .........................................................................................325 mg
- *10 mg oxycodone HCl is equivalent to 8.9637 mg of oxycodone.
All strengths of oxycodone and acetaminophen tablets USP also contain the following inactive ingredients: crospovidone, microcrystalline cellulose, povidone, pregelatinized starch, silicon dioxide and stearic acid.
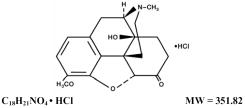
Oxycodone, 4,5α-epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one hydrochloride, is a semisynthetic opioid analgesic which occurs as a white, odorless, crystalline powder having a saline, bitter taste. It is derived from the opium alkaloid thebaine. Oxycodone hydrochloride may be represented by the following structural formula:
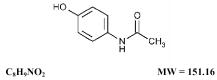
Acetaminophen, 4'-hydroxyacetanilide, is a non-opiate, non-salicylate analgesic and antipyretic which occurs as a white, odorless, crystalline powder, possessing a slightly bitter taste. It may be represented by the following structural formula:
INDICATIONS AND USAGE
Oxycodone and acetaminophen tablets USP are indicated for the relief of moderate to moderately severe pain.
CONTRAINDICATIONS
Oxycodone and acetaminophen tablets should not be administered to patients with known hypersensitivity to oxycodone, acetaminophen, or any other component of this product.
Oxycodone is contraindicated in any situation where opioids are contraindicated including patients with significant respiratory depression (in unmonitored settings or the absence of resuscitative equipment) and patients with acute or severe bronchial asthma or hypercarbia. Oxycodone is contraindicated in the setting of suspected or known paralytic ileus.
General
Opioid analgesics should be used with caution when combined with CNS depressant drugs, and should be reserved for cases where the benefits of opioid analgesia outweigh the known risks of respiratory depression, altered mental state, and postural hypotension.
Information for Patients/Caregivers
The following information should be provided to patients receiving oxycodone and acetaminophen tablets by their physician, nurse, pharmacist, or caregiver:
- Patients should be aware that oxycodone and acetaminophen tablets contain oxycodone, which is a morphine-like substance.
- Patients should be instructed to keep oxycodone and acetaminophen tablets in a secure place out of the reach of children. In the case of accidental ingestions, emergency medical care should be sought immediately.
- When oxycodone and acetaminophen tablets are no longer needed, the unused tablets should be destroyed by flushing down the toilet.
- Patients should be advised not to adjust the medication dose themselves. Instead, they must consult with their prescribing physician.
- Patients should be advised that oxycodone and acetaminophen tablets may impair mental and/or physical ability required for the performance of potentially hazardous tasks (e.g., driving, operating heavy machinery).
- Patients should not combine oxycodone and acetaminophen tablets with alcohol, opioid analgesics, tranquilizers, sedatives, or other CNS depressants unless under the recommendation and guidance of a physician. When co-administered with another CNS depressant, oxycodone and acetaminophen tablets can cause dangerous additive central nervous system or respiratory depression, which can result in serious injury or death.
- The safe use of oxycodone and acetaminophen tablets during pregnancy has not been established; thus, women who are planning to become pregnant or are pregnant should consult with their physician before taking oxycodone and acetaminophen tablets.
- Nursing mothers should consult with their physicians about whether to discontinue nursing or discontinue oxycodone and acetaminophen tablets because of the potential for serious adverse reactions to nursing infants.
- Patients who are treated with oxycodone and acetaminophen tablets for more than a few weeks should be advised not to abruptly discontinue the medication. Patients should consult with their physician for a gradual discontinuation dose schedule to taper off the medication.
- Patients should be advised that oxycodone and acetaminophen tablets are a potential drug of abuse. They should protect it from theft, and it should never be given to anyone other than the individual for whom it was prescribed.
Laboratory Tests
Although oxycodone may cross-react with some drug urine tests, no available studies were found which determined the duration of detectability of oxycodone in urine drug screens. However, based on pharmacokinetic data, the approximate duration of detectability for a single dose of oxycodone is roughly estimated to be one to two days following drug exposure.
Urine testing for opiates may be performed to determine illicit drug use and for medical reasons such as evaluation of patients with altered states of consciousness or monitoring efficacy of drug rehabilitation efforts. The preliminary identification of opiates in urine involves the use of an immunoassay screening and thin-layer chromatography (TLC). Gas chromatography/mass spectrometry (GC/MS) may be utilized as a third-stage identification step in the medical investigational sequence for opiate testing after immunoassay and TLC. The identities of 6-keto opiates (e.g., oxycodone) can further be differentiated by the analysis of their methoxime-trimethylsilyl (MO-TMS) derivative.
Drug/Laboratory Test Interactions
Depending on the sensitivity/specificity and the test methodology, the individual components of oxycodone and acetaminophen tablets may cross-react with assays used in the preliminary detection of cocaine (primary urinary metabolite, benzoylecgonine) or marijuana (cannabinoids) in human urine. A more specific alternate chemical method must be used in order to obtain a confirmed analytical result. The preferred confirmatory method is gas chromatography/mass spectrometry (GC/MS). Moreover, clinical considerations and professional judgment should be applied to any drug-of-abuse test result, particularly when preliminary positive results are used.
Acetaminophen may interfere with home blood glucose measurement systems; decreases of > 20% in mean glucose values may be noted. This effect appears to be drug, concentration and system dependent.
Teratogenic Effects. Pregnancy Category C – Animal reproductive studies have not been conducted with oxycodone and acetaminophen. It is also not known whether oxycodone and acetaminophen tablets can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Oxycodone and acetaminophen tablets should not be given to a pregnant woman unless in the judgment of the physician, the potential benefits outweigh the possible hazards.
Nonteratogenic Effects – Opioids can cross the placental barrier and have the potential to cause neonatal respiratory depression. Opioid use during pregnancy may result in a physically drug-dependent fetus. After birth, the neonate may suffer severe withdrawal symptoms.
Labor and Delivery
Oxycodone and acetaminophen tablets are not recommended for use in women during and immediately prior to labor and delivery due to its potential effects on respiratory function in the newborn.
Nursing Mothers
Ordinarily, nursing should not be undertaken while a patient is receiving oxycodone and acetaminophen tablets because of the possibility of sedation and/or respiratory depression in the infant. Oxycodone is excreted in breast milk in low concentrations, and there have been rare reports of somnolence and lethargy in babies of nursing mothers taking an oxycodone/acetaminophen product. Acetaminophen is also excreted in breast milk in low concentrations.
Geriatric Use
Special precaution should be given when determining the dosing amount and frequency of oxycodone and acetaminophen tablets for geriatric patients, since clearance of oxycodone may be slightly reduced in this patient population when compared to younger patients.
ADVERSE REACTIONS
Serious adverse reactions that may be associated with oxycodone and acetaminophen tablet use include respiratory depression, apnea, respiratory arrest, circulatory depression, hypotension, and shock (see OVERDOSAGE).
The most frequently observed non-serious adverse reactions include lightheadedness, dizziness, drowsiness or sedation, nausea, and vomiting. These effects seem to be more prominent in ambulatory than in nonambulatory patients, and some of these adverse reactions may be alleviated if the patient lies down. Other adverse reactions include euphoria, dysphoria, constipation, and pruritus.
Hypersensitivity reactions may include: Skin eruptions, urticarial, erythematous skin reactions. Hematologic reactions may include: Thrombocytopenia, neutropenia, pancytopenia, hemolytic anemia. Rare cases of agranulocytosis have likewise been associated with acetaminophen use. In high doses, the most serious adverse effect is a dose-dependent, potentially fatal hepatic necrosis. Renal tubular necrosis and hypoglycemic coma also may occur.
Other adverse reactions obtained from postmarketing experiences with oxycodone and acetaminophen tablets are listed by organ system and in decreasing order of severity and/or frequency as follows:
Body as a Whole
Anaphylactoid reaction, allergic reaction, malaise, asthenia, fatigue, chest pain, fever, hypothermia, thirst, headache, increased sweating, accidental overdose, non-accidental overdose
Cardiovascular
Hypotension, hypertension, tachycardia, orthostatic hypotension, bradycardia, palpitations, dysrhythmias
Central and Peripheral Nervous System
Stupor, tremor, paraesthesia, hypoaesthesia, lethargy, seizures, anxiety, mental impairment, agitation, cerebral edema, confusion, dizziness
Fluid and Electrolyte
Dehydration, hyperkalemia, metabolic acidosis, respiratory alkalosis
Gastrointestinal
Dyspepsia, taste disturbances, abdominal pain, abdominal distention, sweating increased, diarrhea, dry mouth, flatulence, gastro-intestinal disorder, nausea, vomiting, pancreatitis, intestinal obstruction, ileus
Hepatic
Transient elevations of hepatic enzymes, increase in bilirubin, hepatitis, hepatic failure, jaundice, hepatotoxicity, hepatic disorder
Hearing and Vestibular
Hearing loss, tinnitus
Hematologic
Thrombocytopenia
Hypersensitivity
Acute anaphylaxis, angioedema, asthma, bronchospasm, laryngeal edema, urticaria, anaphylactoid reaction
Metabolic and Nutritional
Hypoglycemia, hyperglycemia, acidosis, alkalosis
Musculoskeletal
Myalgia, rhabdomyolysis
Ocular
Miosis, visual disturbances, red eye
Psychiatric
Drug dependence, drug abuse, insomnia, confusion, anxiety, agitation, depressed level of consciousness, nervousness, hallucination, somnolence, depression, suicide
Respiratory System
Bronchospasm, dyspnea, hyperpnea, pulmonary edema, tachypnea, aspiration, hypoventilation, laryngeal edema
Skin and Appendages
Erythema, urticaria, rash, flushing
Urogenital
Interstitial nephritis, papillary necrosis, proteinuria, renal insufficiency and failure, urinary retention
DRUG ABUSE AND DEPENDENCE
Oxycodone and acetaminophen tablets are a Schedule II controlled substance. Oxycodone is a mu-agonist opioid with an abuse liability similar to morphine. Oxycodone, like morphine and other opioids used in analgesia, can be abused and is subject to criminal diversion.
Drug addiction is defined as an abnormal, compulsive use, use for non-medical purposes of a substance despite physical, psychological, occupational or interpersonal difficulties resulting from such use, and continued use despite harm or risk of harm. Drug addiction is a treatable disease, utilizing a multi-disciplinary approach, but relapse is common. Opioid addiction is relatively rare in patients with chronic pain but may be more common in individuals who have a past history of alcohol or substance abuse or dependence. Pseudoaddiction refers to pain relief seeking behavior of patients whose pain is poorly managed. It is considered an iatrogenic effect of ineffective pain management. The health care provider must assess continuously the psychological and clinical condition of a pain patient in order to distinguish addiction from pseudoaddiction and thus, be able to treat the pain adequately.
Physical dependence on a prescribed medication does not signify addiction. Physical dependence involves the occurrence of a withdrawal syndrome when there is sudden reduction or cessation in drug use or if an opiate antagonist is administered. Physical dependence can be detected after a few days of opioid therapy. However, clinically significant physical dependence is only seen after several weeks of relatively high dosage therapy. In this case, abrupt discontinuation of the opioid may result in a withdrawal syndrome. If the discontinuation of opioids is therapeutically indicated, gradual tapering of the drug over a 2-week period will prevent withdrawal symptoms. The severity of the withdrawal syndrome depends primarily on the daily dosage of the opioid, the duration of therapy and medical status of the individual.
The withdrawal syndrome of oxycodone is similar to that of morphine. This syndrome is characterized by yawning, anxiety, increased heart rate and blood pressure, restlessness, nervousness, muscle aches, tremor, irritability, chills alternating with hot flashes, salivation, anorexia, severe sneezing, lacrimation, rhinorrhea, dilated pupils, diaphoresis, piloerection, nausea, vomiting, abdominal cramps, diarrhea and insomnia, and pronounced weakness and depression.
“Drug-seeking” behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated “loss” of prescriptions, tampering with prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). “Doctor Shopping” to obtain additional prescriptions is common among drug abusers and people suffering from untreated infection.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of true addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. Oxycodone, like other opioids, has been diverted for non-medical use. Careful record-keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Like other opioid medications, oxycodone and acetaminophen tablets are subject to the Federal Controlled Substances Act. After chronic use, oxycodone and acetaminophen tablets should not be discontinued abruptly when it is thought that the patient has become physically dependent on oxycodone.
DOSAGE AND ADMINISTRATION
Dosage should be adjusted according to the severity of the pain and the response of the patient. It may occasionally be necessary to exceed the usual dosage recommended below in cases of more severe pain or in those patients who have become tolerant to the analgesic effect of opioids. If pain is constant, the opioid analgesic should be given at regular intervals on an around-the-clock schedule. Oxycodone and acetaminophen tablets are given orally.
The total daily dose of acetaminophen should not exceed 4 grams.
| Strength | Usual Adult Dosage | Maximal Daily Dose |
| Oxycodone and acetaminophen tablets 5 mg/325 mg | 1 tablet every 6 hours as needed for pain | 12 Tablets |
| Oxycodone and acetaminophen tablets 7.5 mg/325 mg | 1 tablet every 6 hours as needed for pain | 8 Tablets |
| Oxycodone and acetaminophen tablets 7.5 mg/500 mg | 1 tablet every 6 hours as needed for pain | 8 Tablets |
| Oxycodone and acetaminophen tablets 10 mg/325 mg | 1 tablet every 6 hours as needed for pain | 6 Tablets |
| OXYCODONE AND ACETAMINOPHEN
oxycodone hydrochloride and acetaminophen tablet |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bryant Ranch Prepack | 171714327 | REPACK(63629-3770) , RELABEL(63629-3770) | |