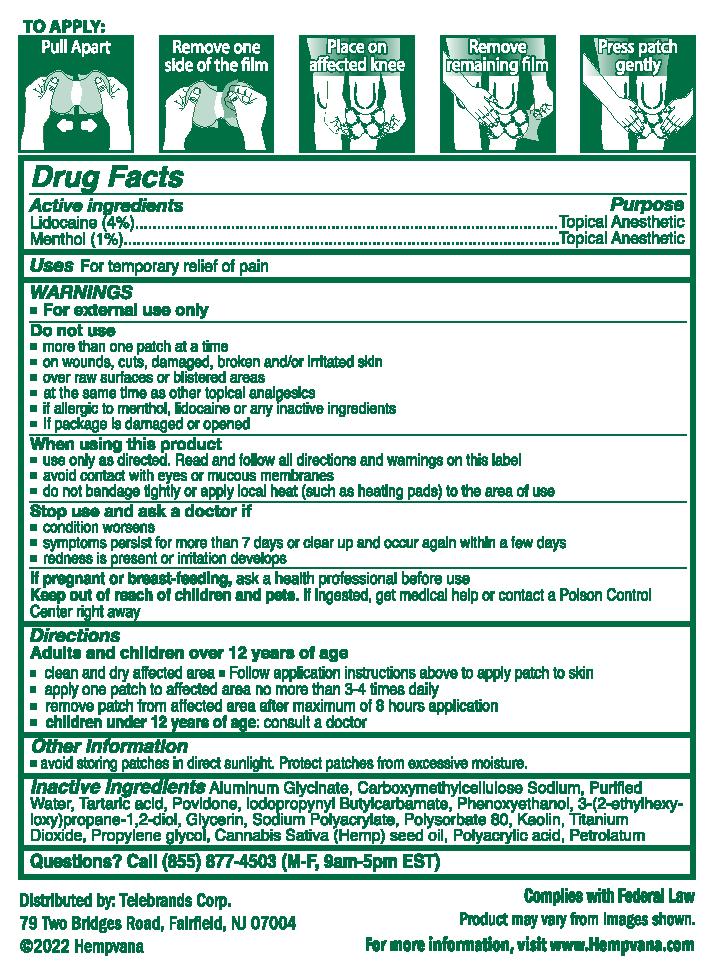
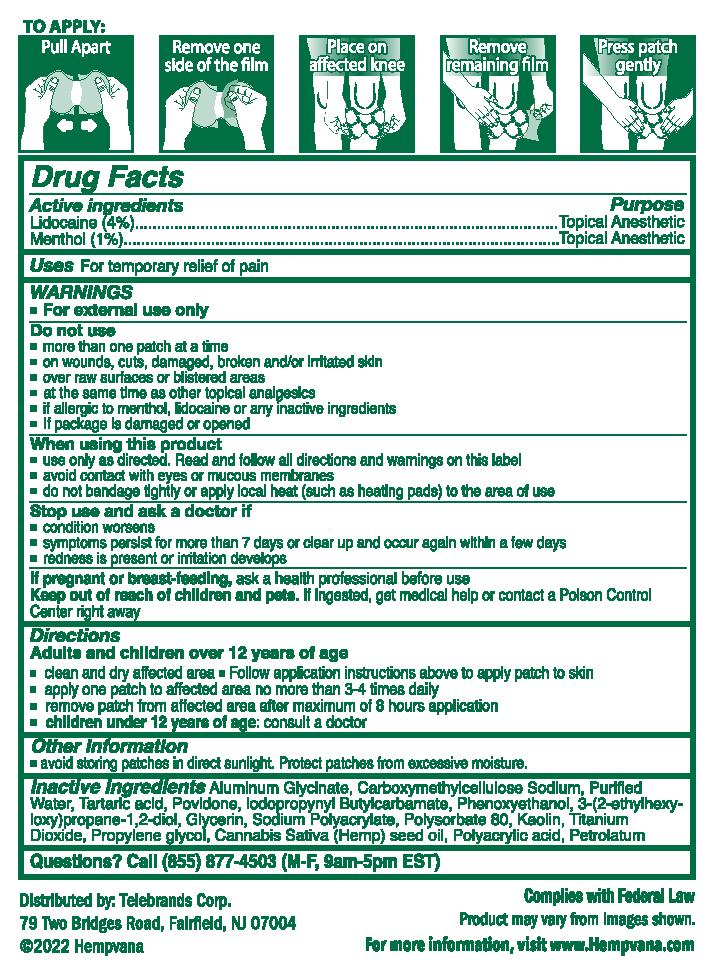
Label: HEMPVANA KNEE BIRD ULTRA STRENGTH PAIN RELIEF- lidocaine, menthol patch
- NDC Code(s): 73287-025-01
- Packager: TELEBRANDS CORP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 27, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- more than one patch at a time
- on wounds, cuts, damaged, broken and/or irritated skin
- over raw surfaces or blistered areas
- at the same time as other topical analgesics
- if allergic to menthol, lidocaine or any other inactive ingredients
- if package is damaged or opened
When using this product
- use only as directed. Read and follow all directions and warnings on this label
- avoid contact with eyes or mucous membranes
- do not bandage tightly or apply local heat (such as heating pads) to the area of use
-
Directions
Adults and children over 12 years of age
- clean and dry affected area
- follow application instructions above to apply patch to skin
- apply one patch to affected area no more than 3-4 times daily
- remove patch from affected area after maximum of 8 hours application
- children under 12 years of age: consult a doctor
- Other information
-
Inactive ingredients
Aluminum Glycinate, Carboxymethylcellulose Sodium, Purified Water, Tartaric acid, Povidone, Iodopropynyl Butylcarbamate, Phenoxyethanol, 3-(2-ethylhexyloxy)propane-1,2-diol, Glycerin, Sodium Polyacrylate, Polysorbate 80, Kaolin, Titanium Dioxide, Propylene Glycol, Cannabis Sativa (Hemp) Seed Oil, Polyacrylic acid, Petrolatum
- Questions?
-
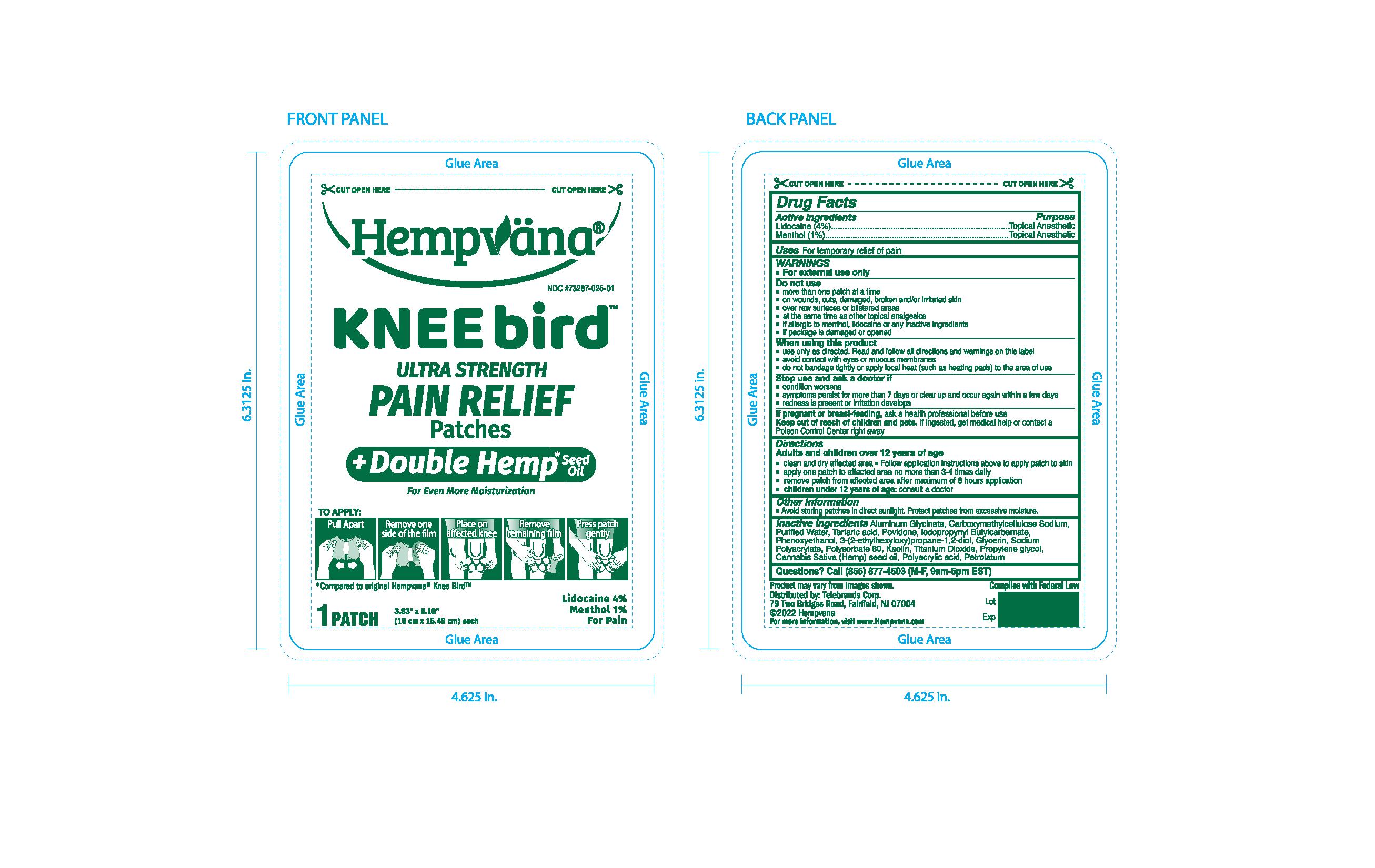
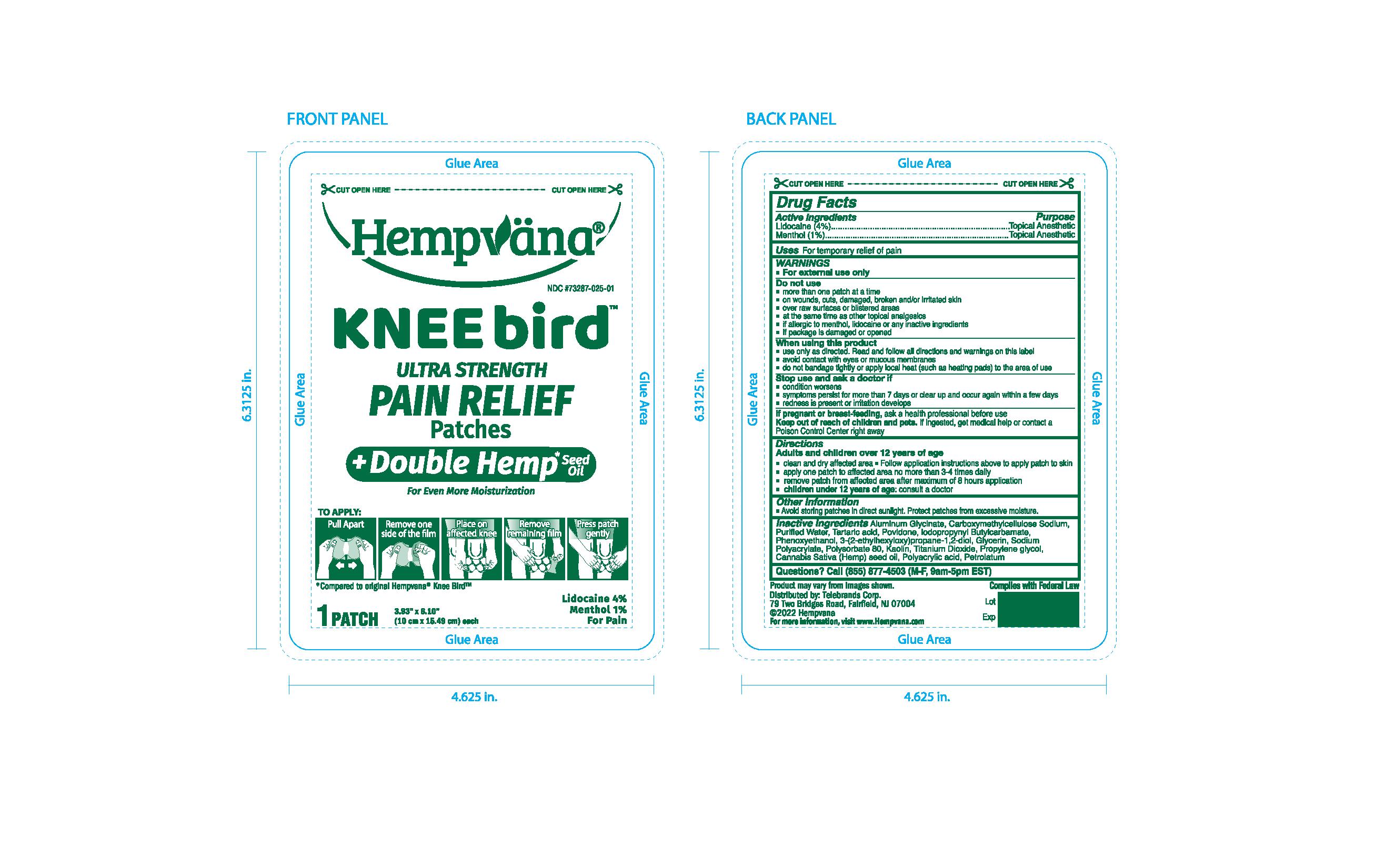
PRINCIPAL DISPLAY PANEL
#1 Hemp Pain Relief Brand in America 1
1AC NIELSEN 2021 Food Drug Mass POS
Pain Relief for Your Knees
Hempvana
Kneebird
Ultra Strength Pain RElief Patches
Bends & Flexes as you move!
As Seen on TV
Not actual size
For Even More Moisturization
Lidocaine 4%
Menthol 1%
For Pain
*Compared to original Hempvana Knee Bird
12 Pourches
Patches 3.93"x6.10"
(10cm x 15.49cm) each

-
INGREDIENTS AND APPEARANCE
HEMPVANA KNEE BIRD ULTRA STRENGTH PAIN RELIEF
lidocaine, menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73287-025 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 100 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength DIHYDROXYALUMINUM AMINOACETATE ANHYDROUS (UNII: 1K713C615K) KAOLIN (UNII: 24H4NWX5CO) POVIDONE (UNII: FZ989GH94E) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) GLYCERIN (UNII: PDC6A3C0OX) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TARTARIC ACID (UNII: W4888I119H) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PHENOXYETHANOL (UNII: HIE492ZZ3T) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73287-025-01 12 in 1 CARTON 05/19/2022 1 11 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 05/19/2022 Labeler - TELEBRANDS CORP (177266558) Establishment Name Address ID/FEI Business Operations Foshan Aqua Gel Biotech Co., Ltd., 529128763 manufacture(73287-025)