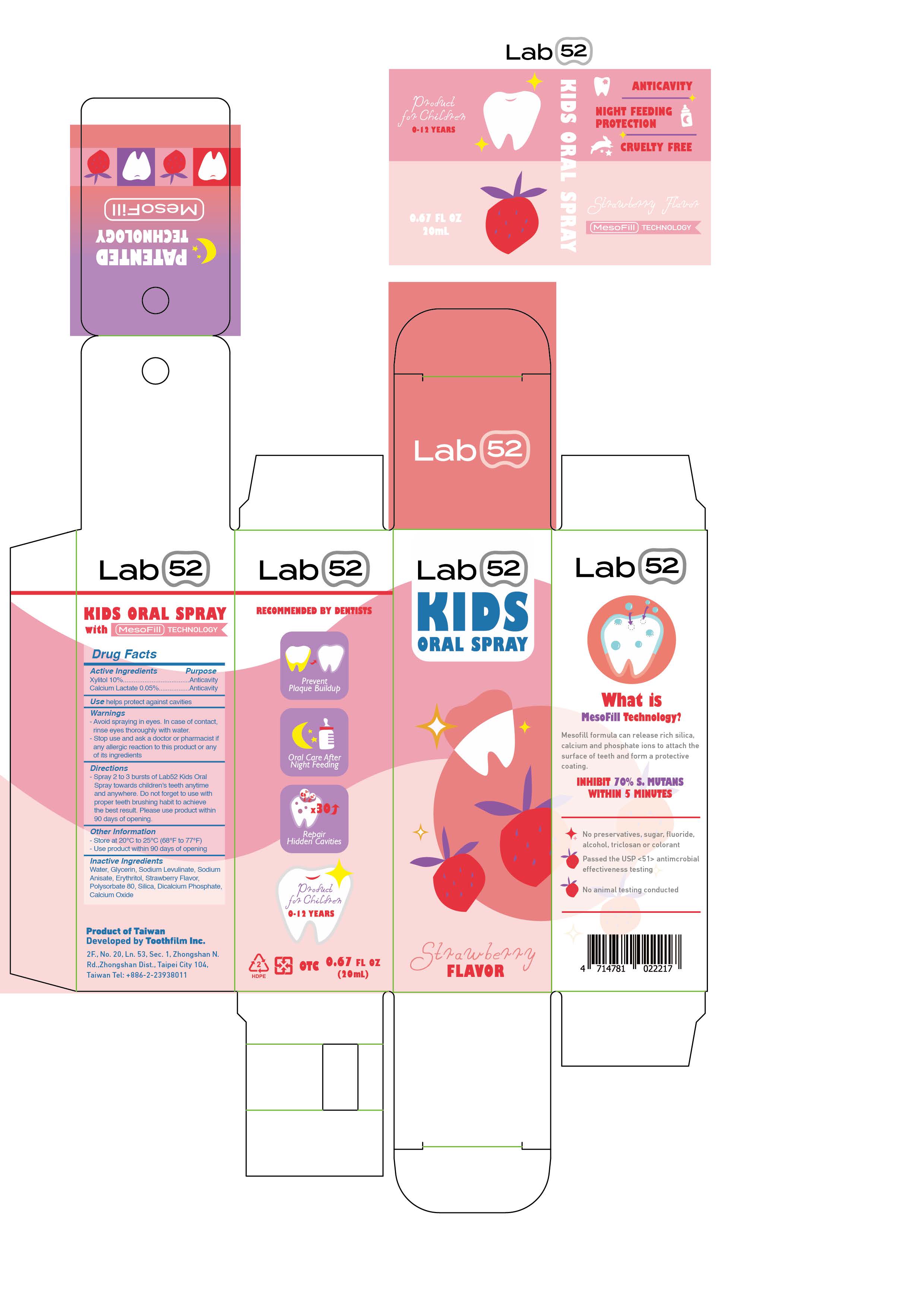
LAB52 KIDS ORAL PROTECTION- xylitol, calcium lactate aerosol
TOOTHFILM INC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
82711-006
Water, Glycerin, Sodium Levulinate, Sodium Anisate, Erythritol, Strawberry Flavor, Polysorbate 80, Silica, Dicalcium Phosphate, Calcium Oxide
- Spray 2 to 3 bursts of Lab52 Kids Oral Spray towards children's teeth anytime and anywhere. Do not forget to use with proper teeth brushing habit to achieve the best result. Please use product within 90 days of opening.
- Avoid spraying in eyes. In case of contact,rinse eyes thoroughly with water.
- Stop use and ask a doctor or pharmacist ifany allergic reaction to this product or anyof its ingredients Directions
Please keep out of reach of children under 6 years old.
Children under six years of age must be supervised by an adult
| LAB52 KIDS ORAL PROTECTION
xylitol, calcium lactate aerosol |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - TOOTHFILM INC (656036549) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TOOTHFILM INC | 656036549 | manufacture(82711-006) | |