Label: BENADRYL- diphenhydramine hydrochloride tablet, film coated
-
NDC Code(s):
66715-6406-2,
66715-6506-2,
66715-9706-1,
66715-9706-2, view more66715-9706-3
- Packager: Lil' Drug Store Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
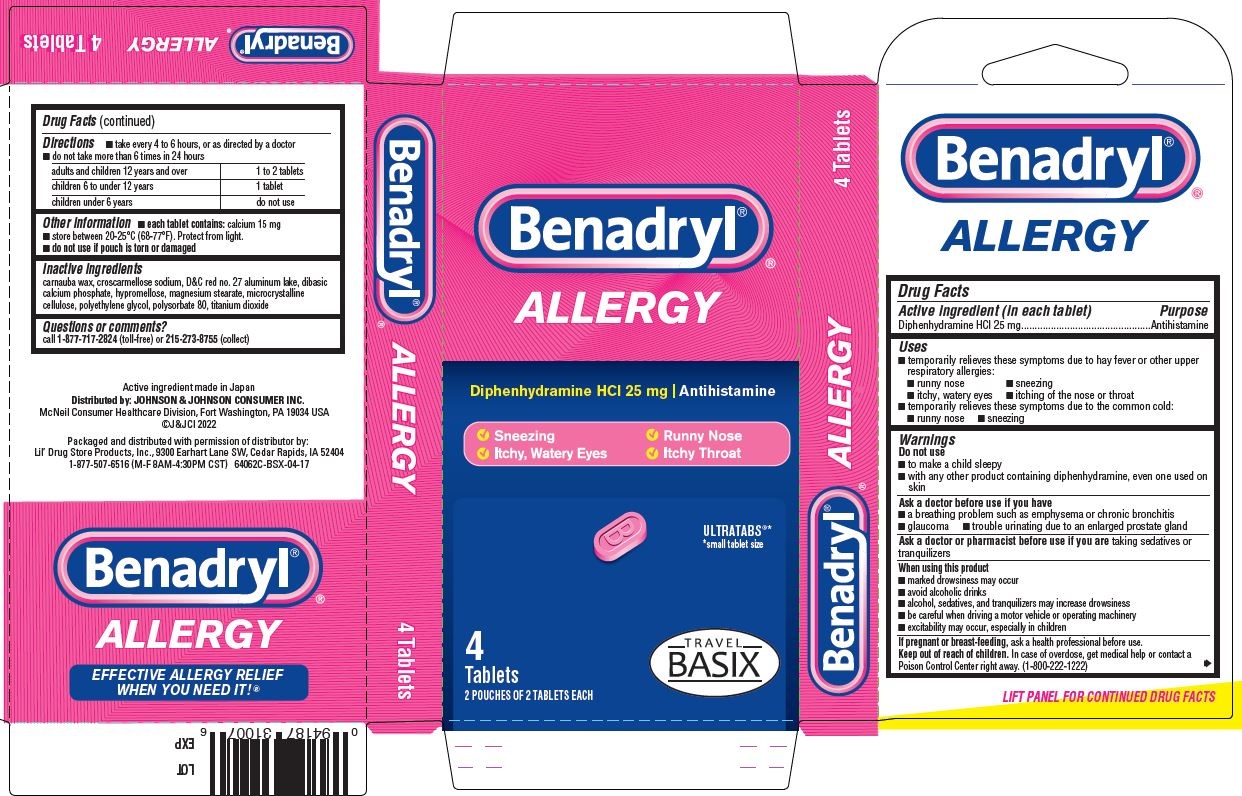
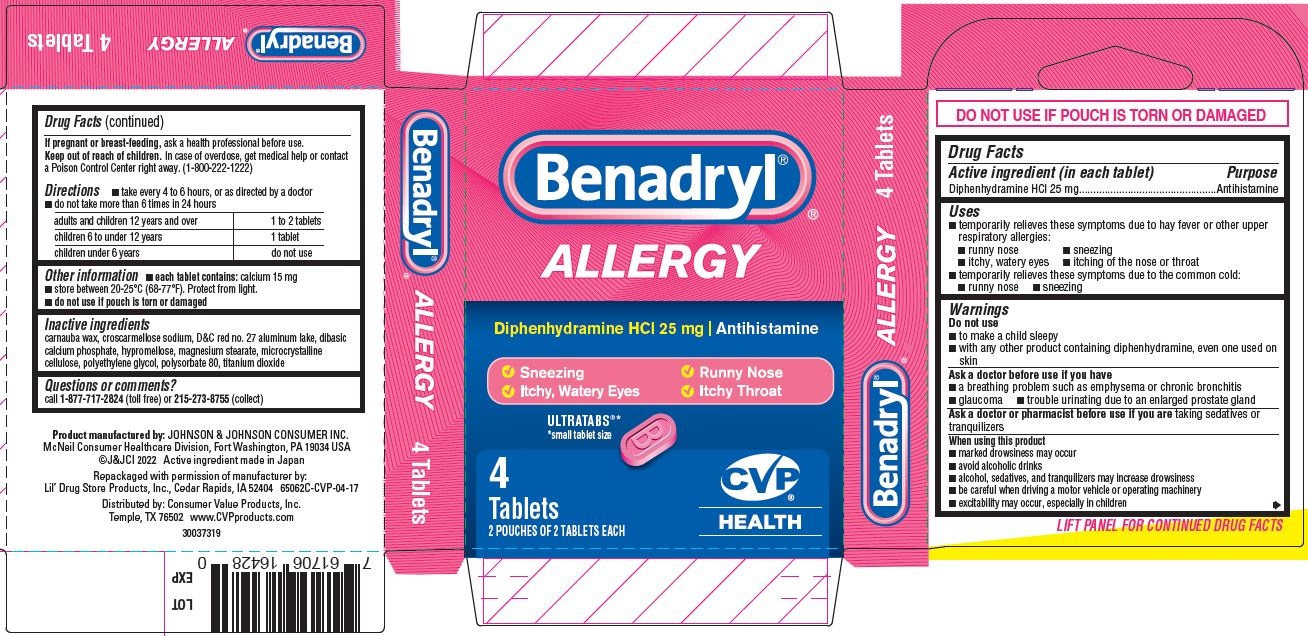
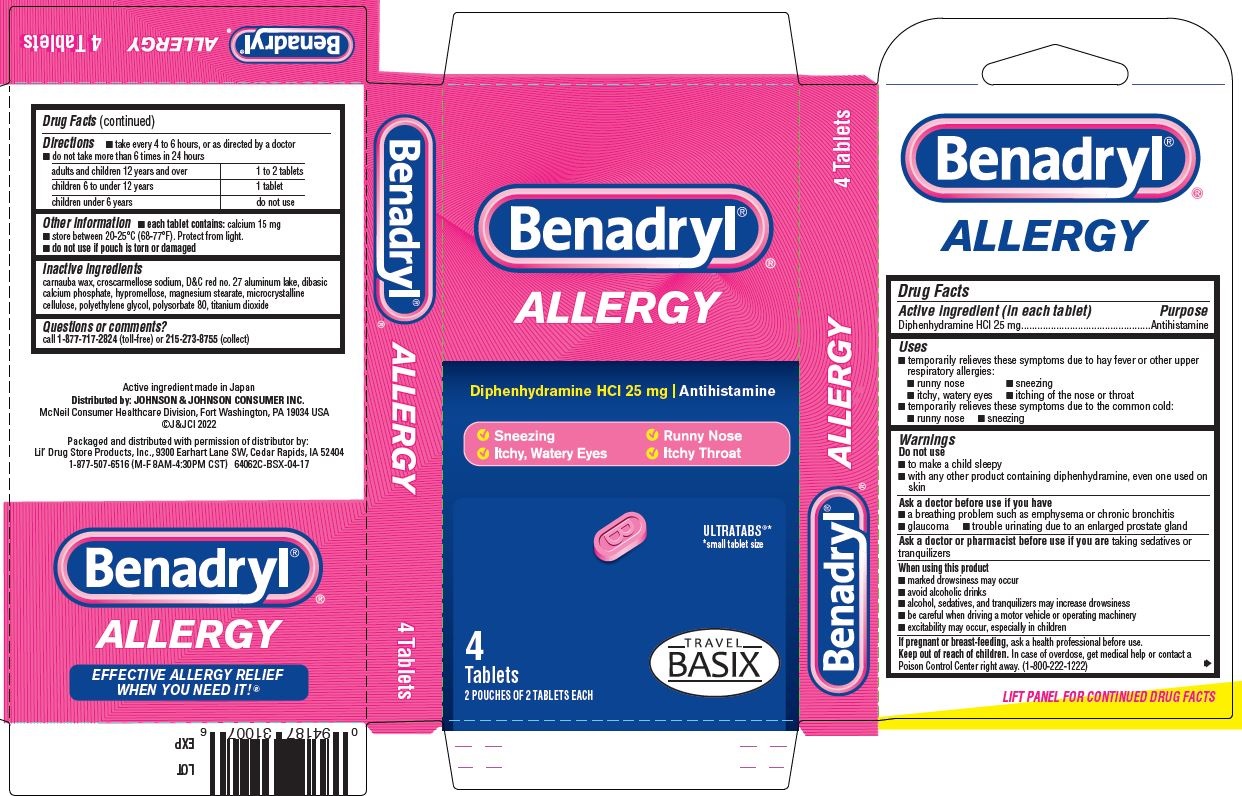
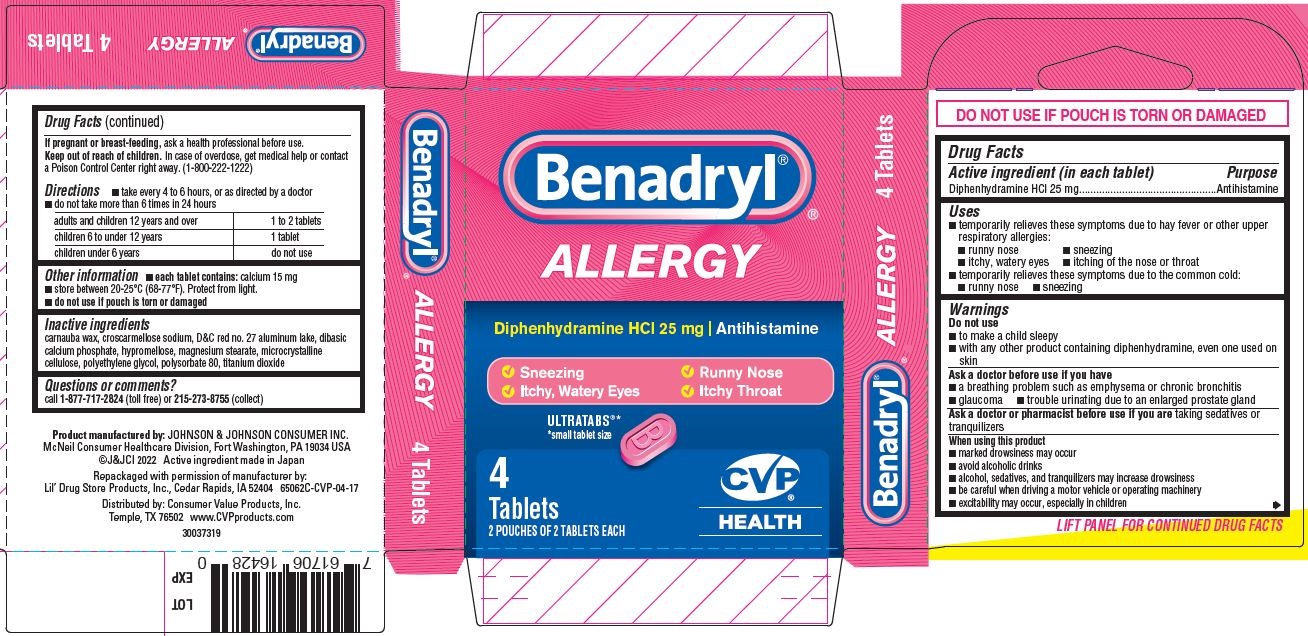
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 25 mg Tablet Pouch Carton
- Benadryl Allergy, Travel BASIX PDP/Package
- Benadryl Allergy, CVP HEALTH PDP/Package
-
INGREDIENTS AND APPEARANCE
BENADRYL
diphenhydramine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66715-9706 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C RED NO. 27 (UNII: 2LRS185U6K) ALUMINUM OXIDE (UNII: LMI26O6933) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color pink Score no score Shape OVAL Size 11mm Flavor Imprint Code B;WL;25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66715-9706-1 1 in 1 CARTON 09/16/2010 07/09/2015 1 2 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:66715-9706-2 2 in 1 CARTON 12/14/2018 2 2 in 1 POUCH; Type 0: Not a Combination Product 3 NDC:66715-9706-3 3 in 1 CARTON 12/14/2018 3 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M012 09/16/2010 BENADRYL
diphenhydramine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66715-6406 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C RED NO. 27 (UNII: 2LRS185U6K) ALUMINUM OXIDE (UNII: LMI26O6933) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color pink Score no score Shape OVAL Size 11mm Flavor Imprint Code B;WL;25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66715-6406-2 2 in 1 CARTON 11/28/2022 1 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M012 11/28/2022 BENADRYL
diphenhydramine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66715-6506 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C RED NO. 27 (UNII: 2LRS185U6K) ALUMINUM OXIDE (UNII: LMI26O6933) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color pink Score no score Shape OVAL Size 11mm Flavor Imprint Code B;WL;25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66715-6506-2 2 in 1 CARTON 05/06/2022 1 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M012 05/06/2022 Labeler - Lil' Drug Store Products, Inc. (093103646)