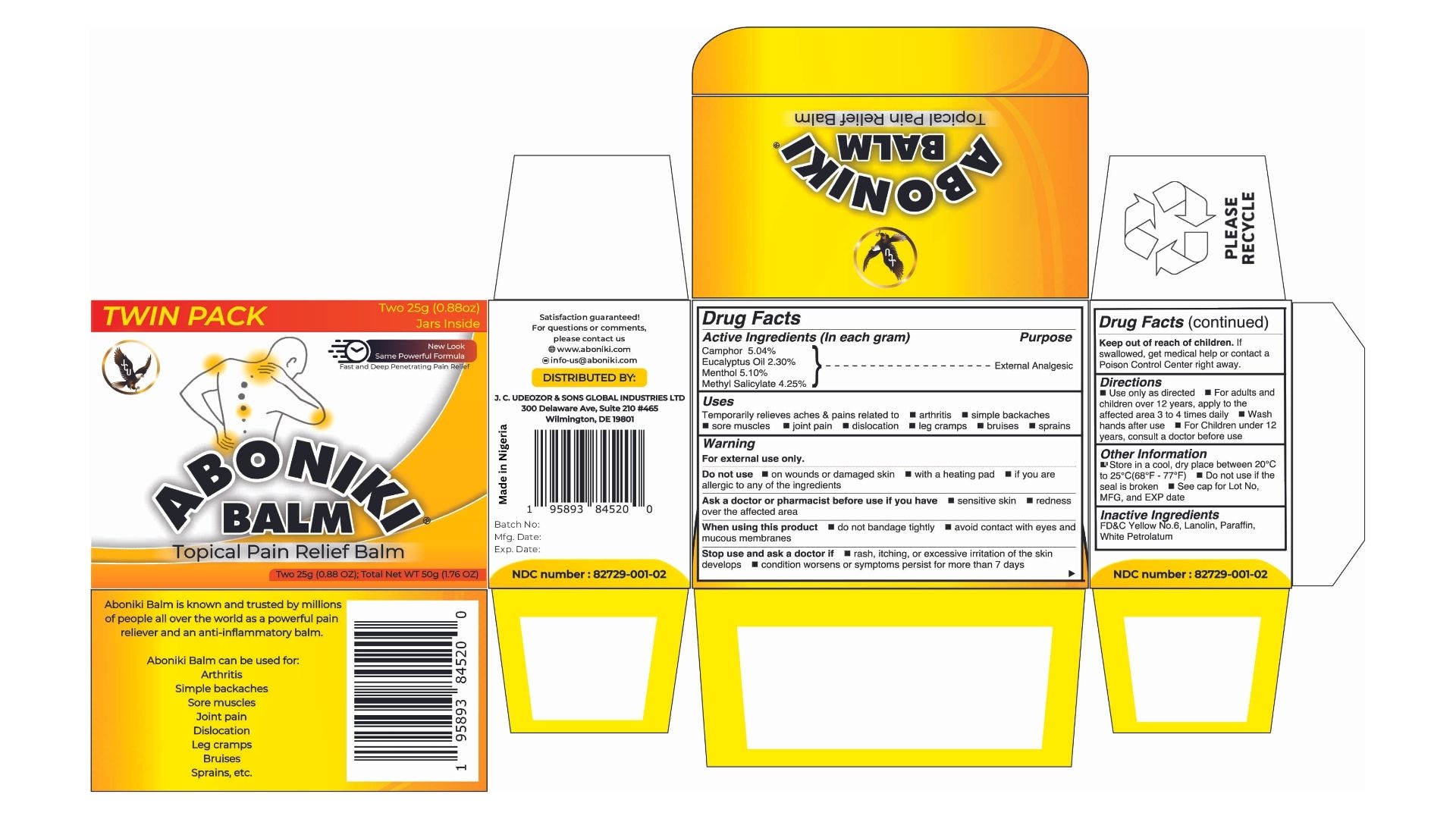
ABONIKI BALM - TOPICAL PAIN RELIEF BALM- camphor, eucalyptus, menthol, methyl salicylate cream
J C UDEOZOR & SONS GLOBAL INDUSTRIES LTD
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients
Active Ingredients (In each gram)
Camphor 5.04%
Eucalyptus Oil 2.30%
Menthol 5.10%
Methyl Salicylate 4.25%
Purpose
| Active Ingredients (In each gram) | Purpose |
| Camphor 5.04% | External Analgesic |
| Eucalyptus Oil 2.30% | External Analgesic |
| Menthol 5.10% | External Analgesic |
| Methyl Salicylate 4.25% | External Analgesic |
USES
Temporarily relieves minor aches & pains related to:
- arthritis
- simple backaches
- sore muscles
- joint pain
- dislocation
- leg cramps
- bruises
- sprains
- lumbago
Do not use
- on wounds or damaged skin
- with a heating pad
- if you are allergic to any of the ingredients
Stop use and ask a doctor if
- rash, itching, or excessive irritation of the skin develops
- condition worsens or symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Direction
- Use only as directed
- For adults and children over 12 years, apply to the affected area 3 to 4 times daily
- Wash hands after use
- For Children under 12 years, consult a doctor before use
Other Information
- Store in a cool, dry place between 20 oC to 25 oC (68 oF - 77 oF)
- Do not use if the seal is broken
- See cap for lot no, mfg, and exp date
| ABONIKI BALM - TOPICAL PAIN RELIEF BALM
camphor, eucalyptus, menthol, methyl salicylate cream |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - J C UDEOZOR & SONS GLOBAL INDUSTRIES LTD (851768299) |
Revised: 7/2023
Document Id: ffadedbd-7446-bdca-e053-6394a90adc8c
Set id: de24a5a5-8780-ee4a-e053-2a95a90a961e
Version: 4
Effective Time: 20230704
J C UDEOZOR & SONS GLOBAL INDUSTRIES LTD