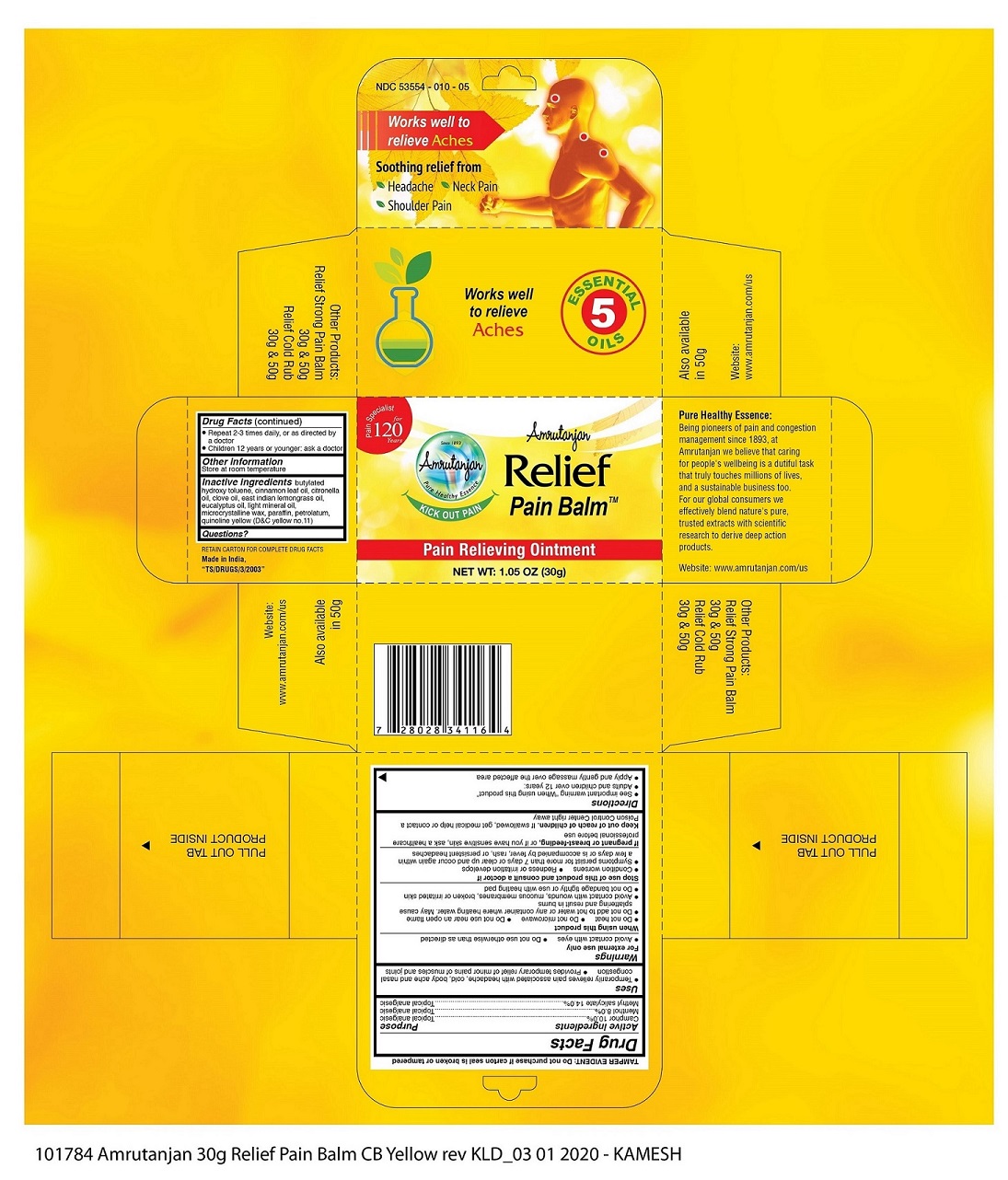
AMRUTANJAN RELIEF PAIN BALM- camphor , menthol, methyl salicylate ointment
AMRUTANJAN HEALTH CARE LIMITED
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
- Temporarily relieves pain associated with headache, cold, body ache and nasal congestion
- Provides temporary relief of minor pains of muscles and joints
When using this product
- Do not heat
- Do not microwave
- Do not use near an open flame
- Do not add to add hot water or any container where heating water. May cause splattering and result in burns
- Avoid contact with wounds, mucous membranes, broken or irritated skin
- Do not bandage tightly or use with heating pad
Stop use this product and consult a doctor if
- Condition worsens
- Redness or irritation develops
- Symptoms persist for more than 7 days or clear up and occur again within a few days or is accompanied by fever, rash, or persistent headaches
Keep out of reach of children, if swallowed, get medical help or contact a poison control center right away
Directions
- See important warning "When using this product"
- Adults and children over 12 years
- Apply and gently massage over the affected area
- Repeat 2 - 3 times daily, or as directed by a doctor
- Children 12 years or younger: ask doctor
| AMRUTANJAN RELIEF PAIN BALM
camphor , menthol, methyl salicylate ointment |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - AMRUTANJAN HEALTH CARE LIMITED (650128309) |
| Registrant - AMRUTANJAN HEALTH CARE LIMITED (650128309) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MAKSON HEALTHCARE PRIVATE LIMITED | 854279690 | manufacture(53554-010) | |
Revised: 9/2022
Document Id: 0041f57a-7563-4cc6-96a6-2ffdd36bd387
Set id: ddee6714-8b18-4d87-944b-8467fda22e6b
Version: 12
Effective Time: 20220919
AMRUTANJAN HEALTH CARE LIMITED