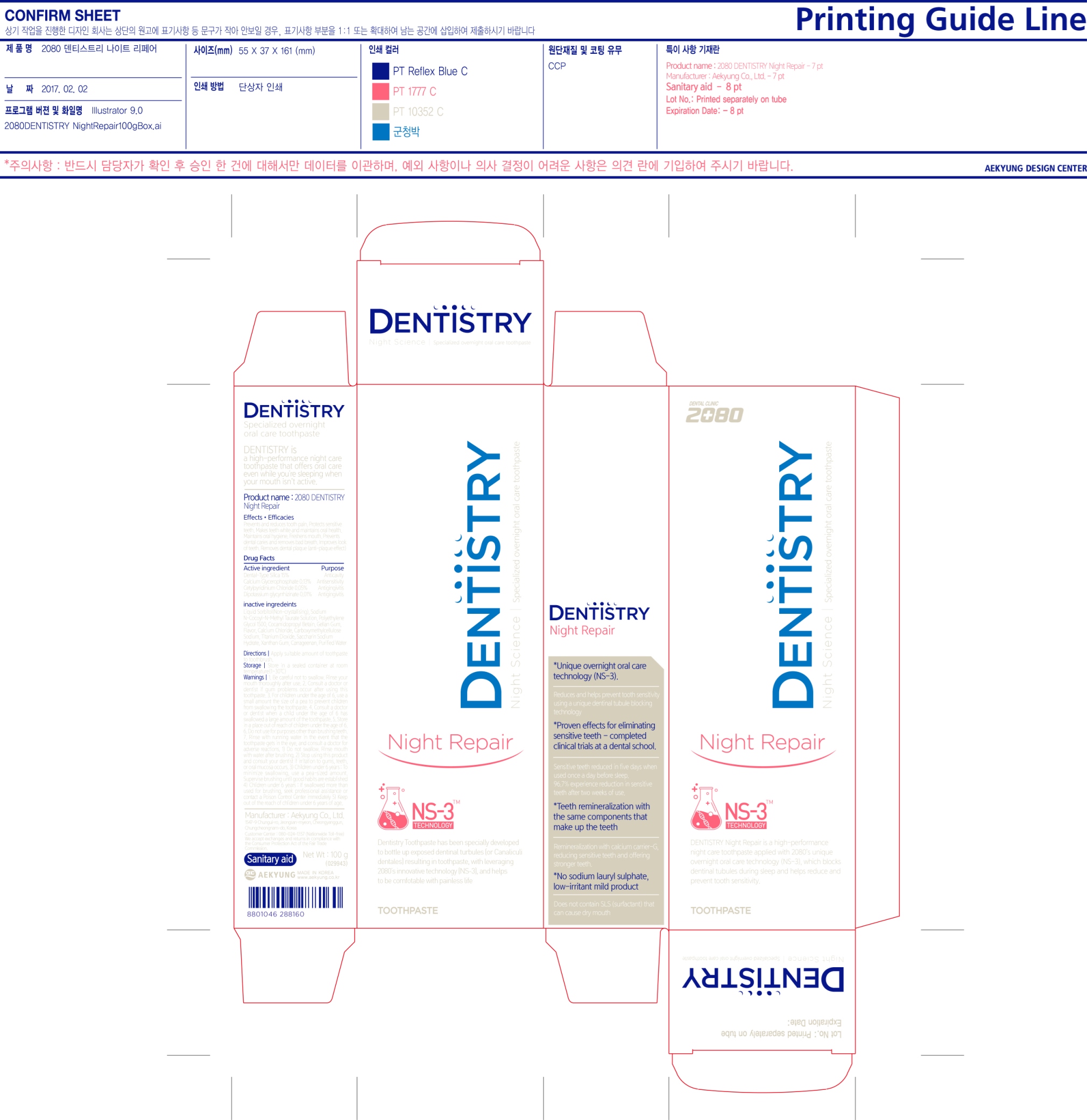
Label: 2080 DENTISTRY NIGHT REPAIR (dental type silica- silicon dioxide, calcium glycerophosphate, cetylpyridinium chloride, dipotassium glycyrrhizinate paste, dentifrice
-
Contains inactivated NDC Code(s)
NDC Code(s): 67225-0010-1 - Packager: Aekyung Industrial Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 3, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
-
DOSAGE & ADMINISTRATION
Do not swallow. Rinse mouth with water after brushing.
Children under 6 years:
- To minimize swallowing, use a pea-sized amount. Supervise brushing until good habits are established.
- If swallowed more than used for brushing, seek professional assistance or contact a Poison Control Center immediately.
- INDICATIONS & USAGE
- INSTRUCTIONS FOR USE
- DO NOT USE
- STORAGE AND HANDLING
- QUESTIONS
- SPL UNCLASSIFIED SECTION
-
INACTIVE INGREDIENT
Inactive Ingredients: Liquid Sorbitol (Non-crystalizing), Sodium N-Cocoyl-N-Methyl Taurate Solution, Polyethylene Glycol 1500, Cocamidopropyl Betain, Gellan Gum, Flavor, Calcium Chloride, Carboxymethylcellulose Sodium, Titanium Dioxide, Saccharin Sodium Hydrate, Xanthan Gum, Carrageenan, Purified Water
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
2080 DENTISTRY NIGHT REPAIR
dental type silica (silicon dioxide), calcium glycerophosphate, cetylpyridinium chloride, dipotassium glycyrrhizinate paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67225-0010 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 15 g in 100 g Inactive Ingredients Ingredient Name Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) CALCIUM GLYCEROPHOSPHATE (UNII: XWV9Z12C1C) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) SORBITOL (UNII: 506T60A25R) SODIUM METHYL COCOYL TAURATE (UNII: JVL98CG53G) POLYETHYLENE GLYCOL 1500 (UNII: 1212Z7S33A) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) SACCHARIN SODIUM (UNII: SB8ZUX40TY) GELLAN GUM (LOW ACYL) (UNII: 7593U09I4D) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) CARRAGEENAN (UNII: 5C69YCD2YJ) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67225-0010-1 1 in 1 CARTON 02/03/2017 1 100 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part332 02/03/2017 Labeler - Aekyung Industrial Co., Ltd. (687995647) Establishment Name Address ID/FEI Business Operations Aekyung Ind. Co., Ltd._Chungyang Factory 690511126 manufacture(67225-0010)