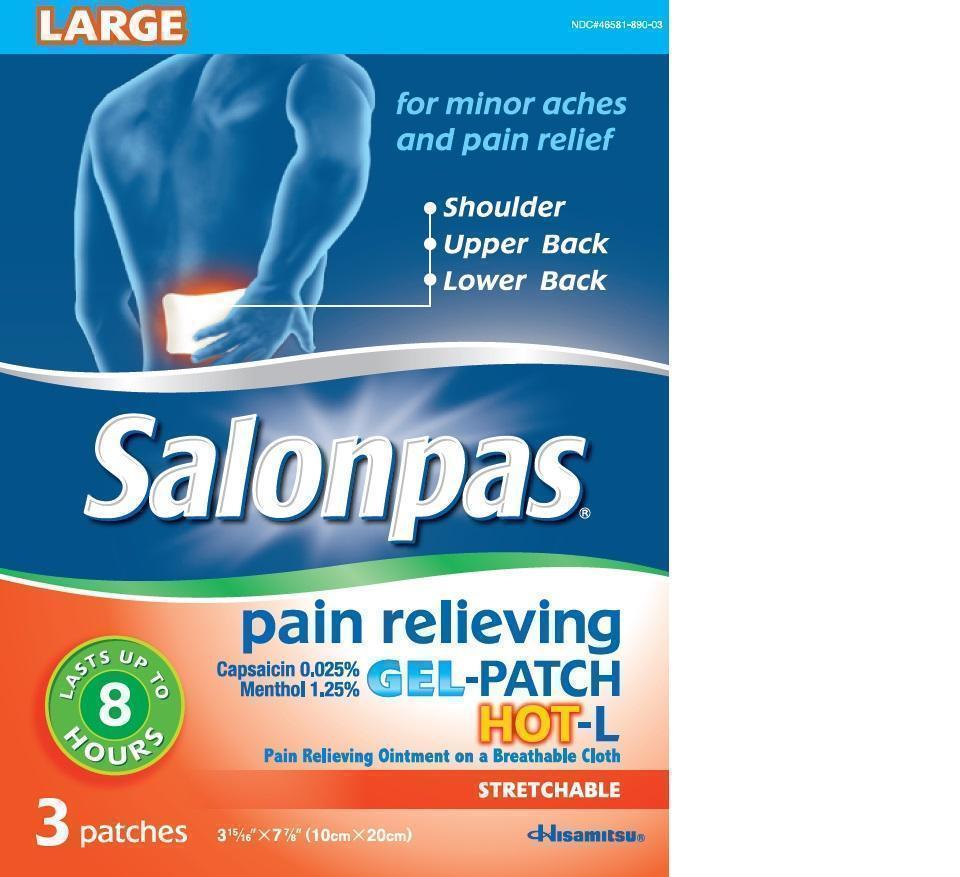
Label: SALONPAS PAIN RELIEVING HOT-L- capsaicin, menthol patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 46581-890-01, 46581-890-03, 46581-890-99 - Packager: Hisamitsu Pharmaceutical Co., Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 27, 2015
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- on wounds or damaged skin
- with a heating pad
- if you are allergic to any ingredients of this product
When using this product
- do not use otherwise than as directed
- avoid contact with the eyes, mucous membranes or rashes
- do not bandage tightly
- discontinue use at least 1 hour before a bath or shower
- do not use immediately after a bath or shower
-
Directions
Adults and children 12 years of age and over:
- clean and dry affected area
- remove film from patch and apply to the skin (see illustration)
- apply to affected area not more than 3 to 4 times daily
- remove patch from the skin after at most 8 hours' application
Children under 12 years of age: consult a doctor
- Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SALONPAS PAIN RELIEVING HOT-L
capsaicin, menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46581-890 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSICUM (UNII: 00UK7646FG) (CAPSICUM - UNII:00UK7646FG) CAPSAICIN 0.025 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1.25 g in 100 g Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) POLYVINYL ALCOHOL (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ALUMINUM SILICATE (UNII: T1FAD4SS2M) EDETATE DISODIUM (UNII: 7FLD91C86K) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) GLYCERIN (UNII: PDC6A3C0OX) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) OLEYL ALCOHOL (UNII: 172F2WN8DV) TARTARIC ACID (UNII: W4888I119H) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46581-890-03 1 in 1 BOX 1 3 in 1 POUCH 1 20 g in 1 PATCH; Type 0: Not a Combination Product 2 NDC:46581-890-01 1 in 1 POUCH 2 20 g in 1 PATCH; Type 0: Not a Combination Product 3 NDC:46581-890-99 1 in 1 POUCH 3 20 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 05/01/2011 Labeler - Hisamitsu Pharmaceutical Co., Inc. (690539713)