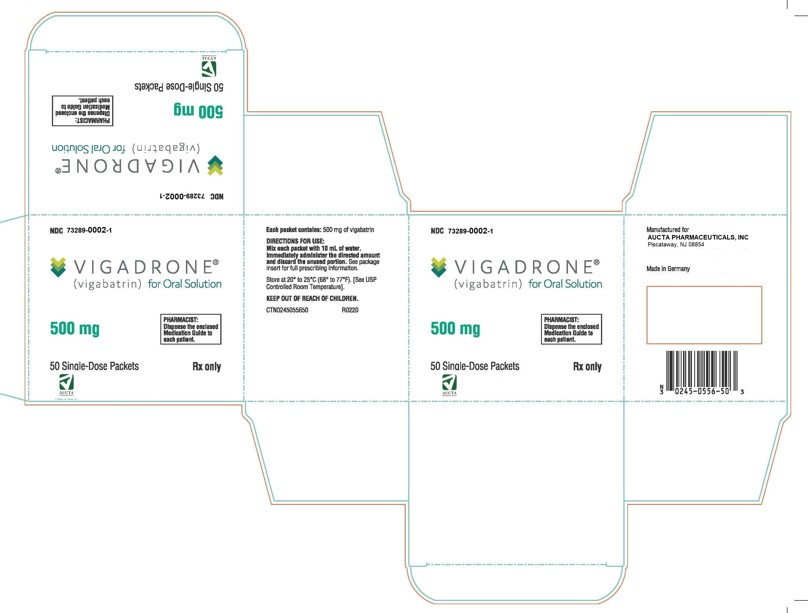
VIGADRONE- vigabatrin powder, for solution
Aucta Pharmaceuticals, Inc.
----------
| VIGADRONE
vigabatrin powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Aucta Pharmaceuticals, Inc. (016894249) |
Revised: 2/2023
Document Id: f4c00841-d1a6-069e-e053-2a95a90a2474
Set id: dd08986d-d172-e930-e053-2a95a90a1ef9
Version: 4
Effective Time: 20230215
Aucta Pharmaceuticals, Inc.