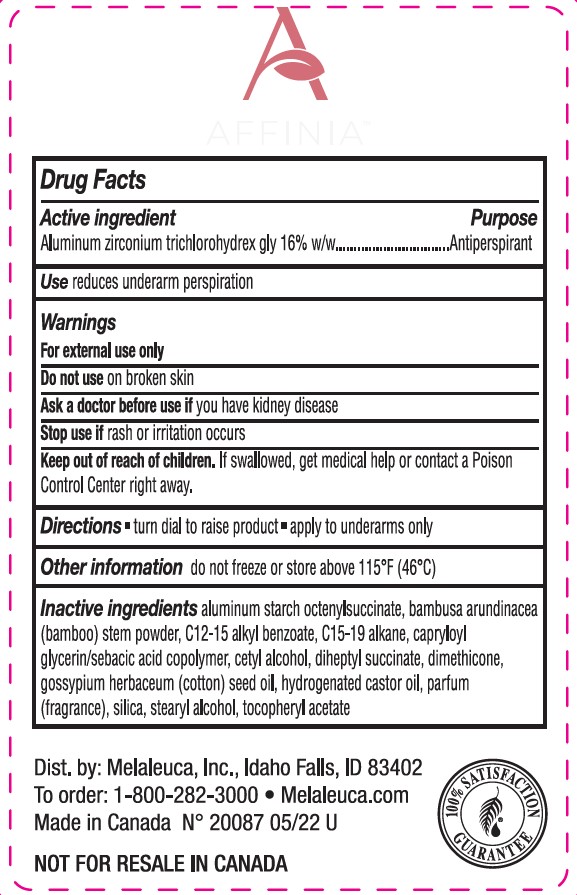
Label: AFFINIA ANTIPERSPIRANT DEODORANT SWEET CREAM AND WILLOW- aluminum zirconium tetrachlorohydrex gly stick
- NDC Code(s): 54473-307-02
- Packager: Melaleuca Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AFFINIA ANTIPERSPIRANT DEODORANT SWEET CREAM AND WILLOW
aluminum zirconium tetrachlorohydrex gly stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54473-307 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY (UNII: 8O386558JE) (ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY - UNII:8O386558JE) ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY 16 g in 57 g Inactive Ingredients Ingredient Name Strength BAMBUSA ARUNDINACEA STEM (UNII: NRA4497HC5) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER (2000 MPA.S) (UNII: N7YC58165T) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DIMETHICONE 100 (UNII: RO266O364U) DIHEPTYL SUCCINATE (UNII: 057N7SS26Y) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) GOSSYPIUM HERBACEUM SEED RESIN (UNII: X408487D9E) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) C15-19 ALKANE (UNII: CI87N1IM01) CETYL ALCOHOL (UNII: 936JST6JCN) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54473-307-02 57 g in 1 APPLICATOR; Type 0: Not a Combination Product 04/12/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 04/12/2022 Labeler - Melaleuca Inc. (139760102) Registrant - Melaleuca Inc. (139760102) Establishment Name Address ID/FEI Business Operations KDC/ONE Development Corporation, Inc 204006464 manufacture(54473-307)