PAIN RELIEVING- pain relieving cream
BluePoint Laboratories
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
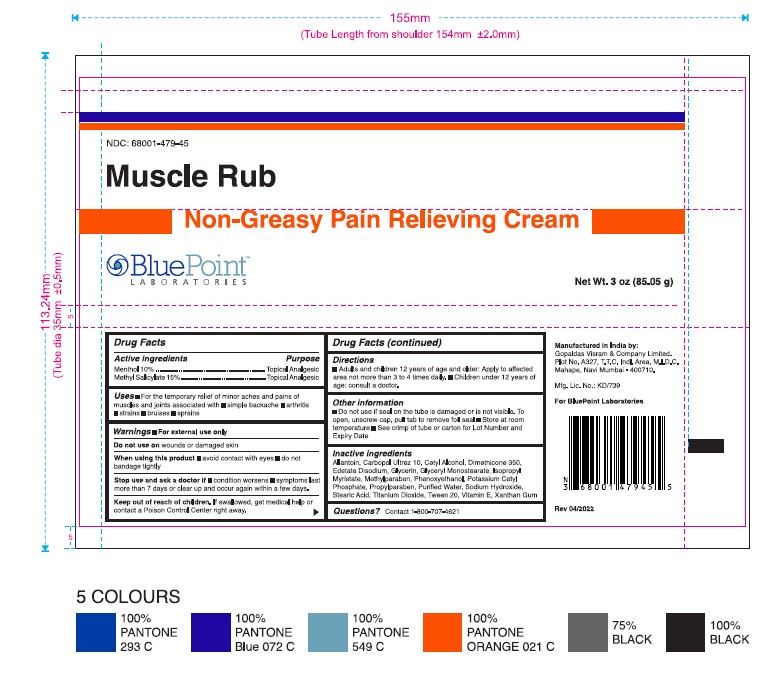
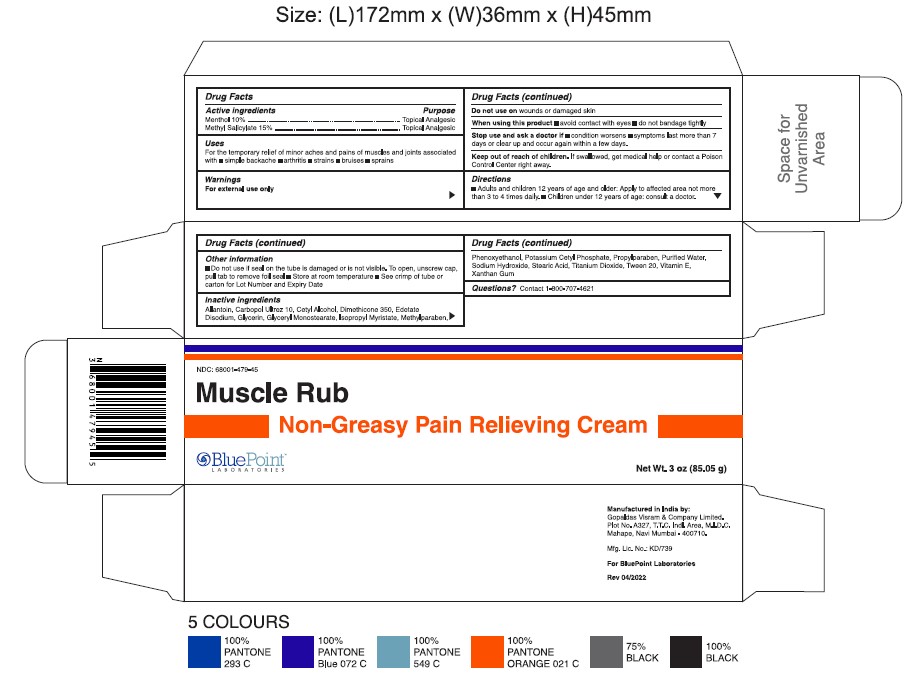
Non-Greasy Pain Relieving Cream (Muscle Rub)
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with
- simple backache
- arthritis
- strains
- bruises
- sprains
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily
- Children under 2 years of age: Consult a doctor
Other Information
- Do not use if seal on the tube is damaged or is not visible. To open, unscrew cap, pull tab to remove foil seal
- Store at room temperature
- See Crimp of tube or carton for Lot Number and Expiry Date
Inactive ingredients
Allantoin, Carbopol Ultrez 10, Cetyl Alcohol, Dimethicone 350, Edetate Disodium, Glycerin, Glyceryl Monostearate, Isopropyl Myristate, Methyl Paraben, Phenoxyethanol, Potassium Cetyl Phosphate, Propyl Paraben, Purified Water, Sodium Hydroxide, Stearic Acid, Titanium Dioxide, Tween 20, Vitamin E, Xanthan Gum
| PAIN RELIEVING
pain relieving cream |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - BluePoint Laboratories (985523874) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Gopaldas Visram & Co., Ltd | 858030888 | manufacture(68001-479) | |
Revised: 9/2023
Document Id: 043b7fd2-ba0b-941f-e063-6394a90a827c
Set id: dc73db77-ab49-49fa-e053-2995a90ae501
Version: 2
Effective Time: 20230904
BluePoint Laboratories