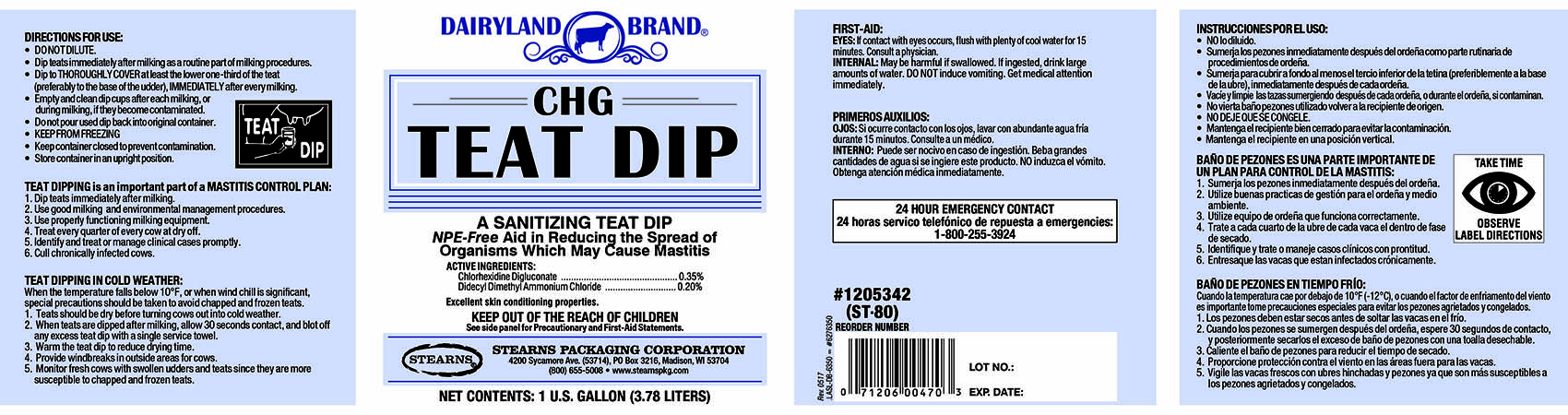
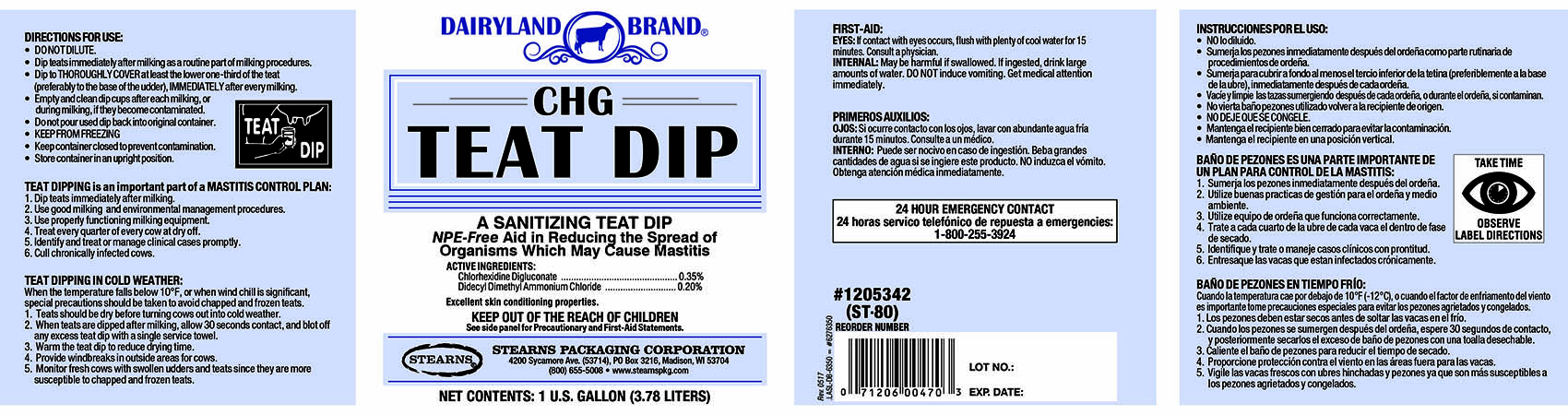
Label: DAIRYLAND BRAND CHG TEAT DIP- chlorhexidine gluconate, didecyldimethylammonium chloride liquid
- NDC Code(s): 60282-2080-1, 60282-2080-2, 60282-2080-3, 60282-2080-4
- Packager: Stearns Packaging Corporation
- Category: OTC ANIMAL DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 7, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
GENERAL PRECAUTIONS
TEAT DIPPING is an important part of a MASTITIS CONTROL PLAN:
1. Dip teats immediately after milking.
2. Use good milking and environmental
management procedures.
3. Use properly functioning milking equipment.
4. Treat every quarter of every cow at dry off.
5. Identify and treat or manage clinical cases
promptly.
6. Cull chronically infected cows.TEAT DIPPING IN COLD WEATHER:
When the temperature falls below 10 degrees F, or when wind chill is significant,
special precautions should be taken to avoid chapped and frozen teats.
1. Teats should be dry before turning cows out into cold weather.
2. When teats are dipped after milking, allow 30 seconds contact, and blot
off any excess teat dip with a single service towel.
3. Warm the teat dip to reduce drying time.
4. Provide windbreaks in outside areas for cows.
5. Monitor fresh cows with swollen udders and teats since they are more
susceptible to chapped and frozen teats. - DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
USER SAFETY WARNINGS
FIRST-AID:
EYES: If contact with eyes occurs, flush with plenty of cool water for 15
minutes. Consult a physician.
INTERNAL: May be harmful if swallowed. If ingested, drink large amounts of
water or milk. DO NOT induce vomiting. Get medical attention immediately.24 HOUR EMERGENCY CONTACT
CHEM-TEL, 1-800-255-3924 -
PRINCIPAL DISPLAY PANEL
DAIRYLAND BRAND
CHG
TEAT DIPA SANITIZING TEAT DIP
NPE-Free Aid in Reducing the Spread of
Organisms Which May Cause Mastitis.ACTIVE INGREDIENTS:
Chlorhexidine Digluconate..........................................0.35%
Didecyl Dimethyl Ammonium Chloride........................0.20%Excellent skin conditioning properties.
KEEP OUT OF THE REACH OF CHILDREN
See side panel for precautionary and First-Aid Statements.STEARNS PACKAGING CORPORATION
4200 Sycamore Ave. (53714), PO Box 3216, Madison, WI 53704
(800) 655-5008 www.stearnspkg.comNET CONTENTS: 1 U.S. FL. GALLON (3.78 LITERS)
-
INGREDIENTS AND APPEARANCE
DAIRYLAND BRAND CHG TEAT DIP
chlorhexidine gluconate, didecyldimethylammonium chloride liquidProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:60282-2080 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 3.5 g in 1 L DIDECYLDIMONIUM CHLORIDE (UNII: JXN40O9Y9B) (DIDECYLDIMONIUM - UNII:Z7F472XQPA) DIDECYLDIMONIUM CHLORIDE 2 g in 1 L Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60282-2080-1 3.78 L in 1 BOTTLE, PLASTIC 2 NDC:60282-2080-2 18.93 L in 1 PAIL 3 NDC:60282-2080-3 56.78 L in 1 DRUM 4 NDC:60282-2080-4 208.2 L in 1 DRUM Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/02/1985 Labeler - Stearns Packaging Corporation (006069256) Registrant - Stearns Packaging Corporation (006069256) Establishment Name Address ID/FEI Business Operations Stearns Packaging Corporation 006069256 manufacture, api manufacture