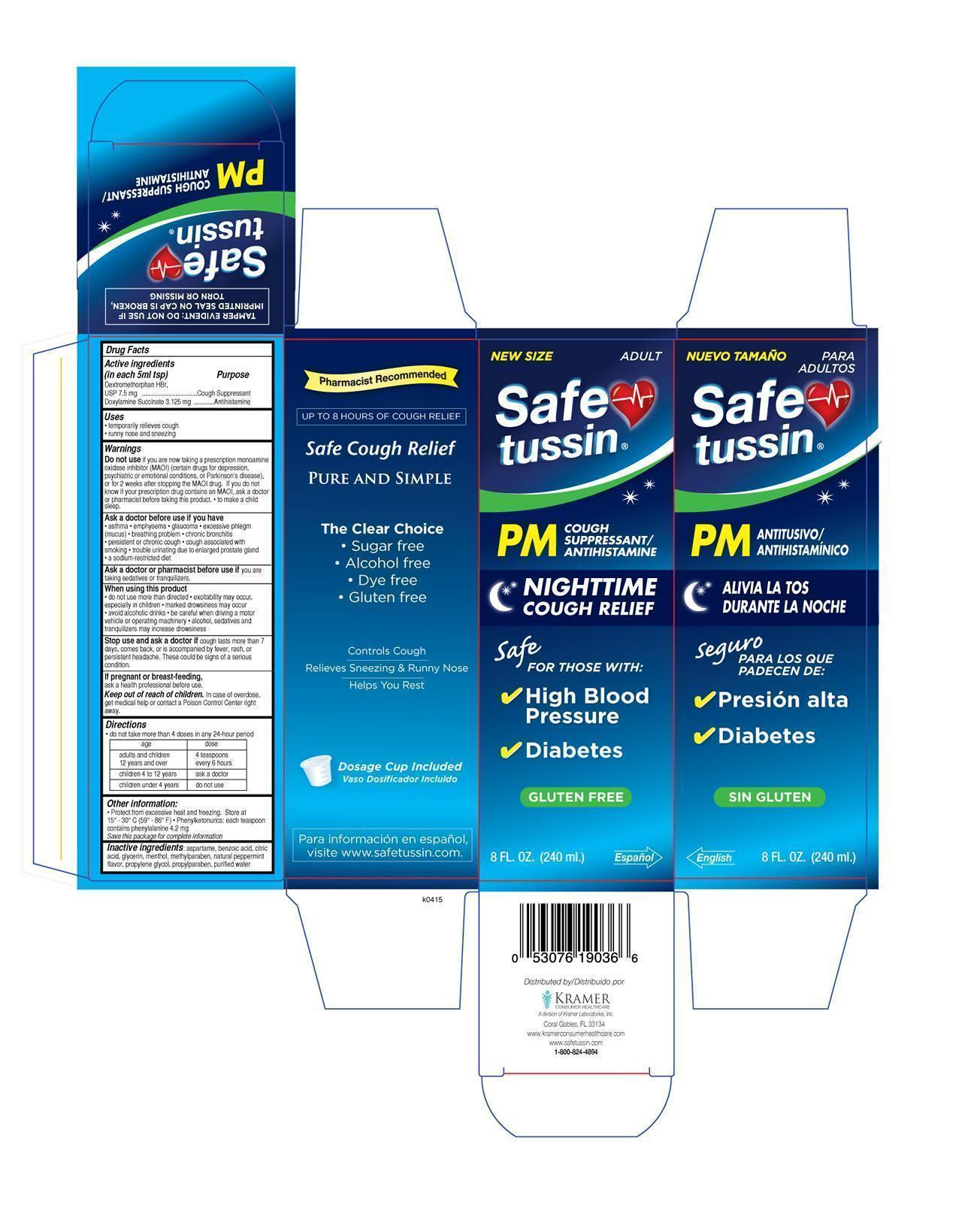
Dextromethorphan
Doxylamine Succinate
Cough Suppressant
Antihistamine
Keep out of reach of children. In case of
overdose, get medical help or contact a Poison
Control Center right away
- temporarily relieves coughgh • runny nose and sneezing
Do not take more than 4 doses in any 24- hours period.
adults and children12 years and over 4 teaspoons every 6 hours
children 4 to 12 years ask a doctor
children under 4 years do not use
aspartame,benzoic acid,citric acid,glycerin,menthol,methylparaben,natural peppermint flavor,propylene glycol,propylparaben
purified water
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product. to make a child sleep.