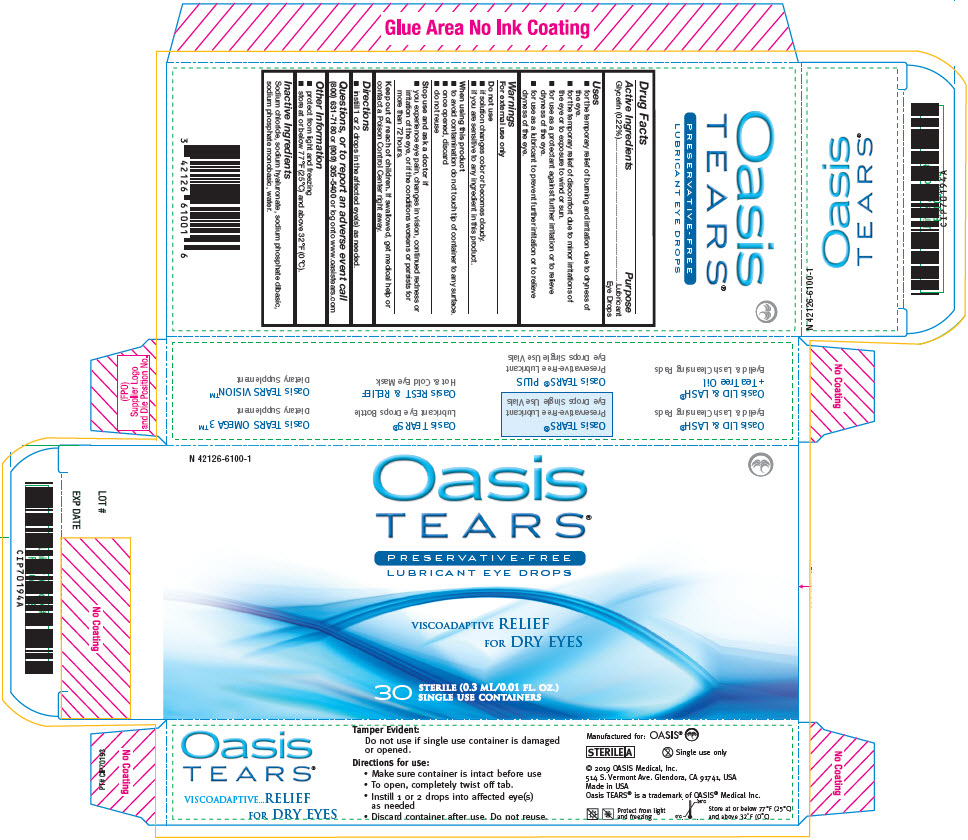
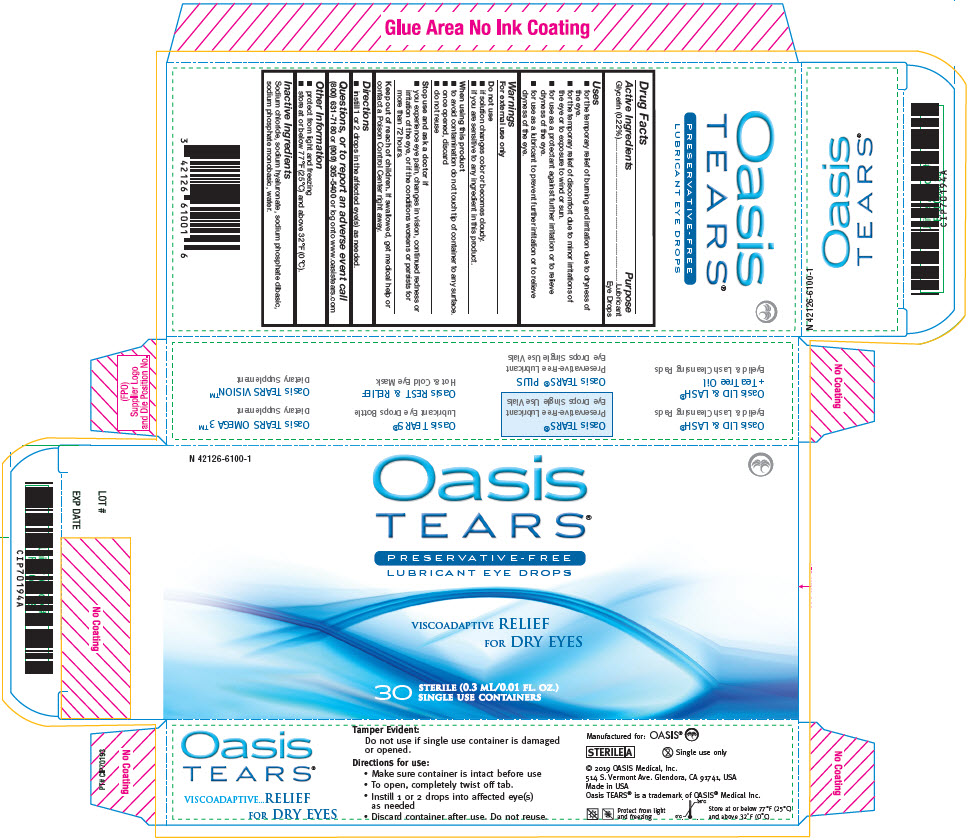
Label: OASIS TEARS PRESERVATIVE-FREE LUBRICANT EYE- glycerin solution/ drops
- NDC Code(s): 42126-6100-1, 42126-6100-2
- Packager: OASIS Medical, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 21, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
-
Uses
- for the temporary relief of burning and irritation due to dryness of the eye.
- for the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun.
- for use as a protectant against further irritation or to relieve dryness of the eye.
- for use as a lubricant to prevent further irritation or to relieve dryness of the eye.
-
Warnings
For external use only
Do not use
- if solution changes color or becomes cloudy.
- if you are sensitive to any ingredient in this product.
When using this product
- to avoid contamination do not touch tip of container to any surface.
- once opened, discard
- do not reuse
- Directions
- Questions, or to report an adverse event call
- Other Information
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL - 0.3 mL Pouch Carton
-
INGREDIENTS AND APPEARANCE
OASIS TEARS PRESERVATIVE-FREE LUBRICANT EYE
glycerin solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42126-6100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Glycerin (UNII: PDC6A3C0OX) (Glycerin - UNII:PDC6A3C0OX) Glycerin .22 g in 100 mL Inactive Ingredients Ingredient Name Strength Hyaluronate Sodium (UNII: YSE9PPT4TH) 0.15 g in 100 mL Sodium chloride (UNII: 451W47IQ8X) Sodium phosphate, monobasic, unspecified form (UNII: 3980JIH2SW) Sodium phosphate, dibasic, unspecified form (UNII: GR686LBA74) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42126-6100-1 3 in 1 CARTON 11/01/2017 1 10 in 1 POUCH 1 0.3 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 2 NDC:42126-6100-2 5 in 1 POUCH 11/01/2017 2 0.3 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 11/01/2007 Labeler - OASIS Medical, Inc. (194121018) Establishment Name Address ID/FEI Business Operations LABORATOIRE UNITHER COUTANCES 574139809 MANUFACTURE(42126-6100) Establishment Name Address ID/FEI Business Operations UNITHER MANUFACTURING LLC 079176615 MANUFACTURE(42126-6100)