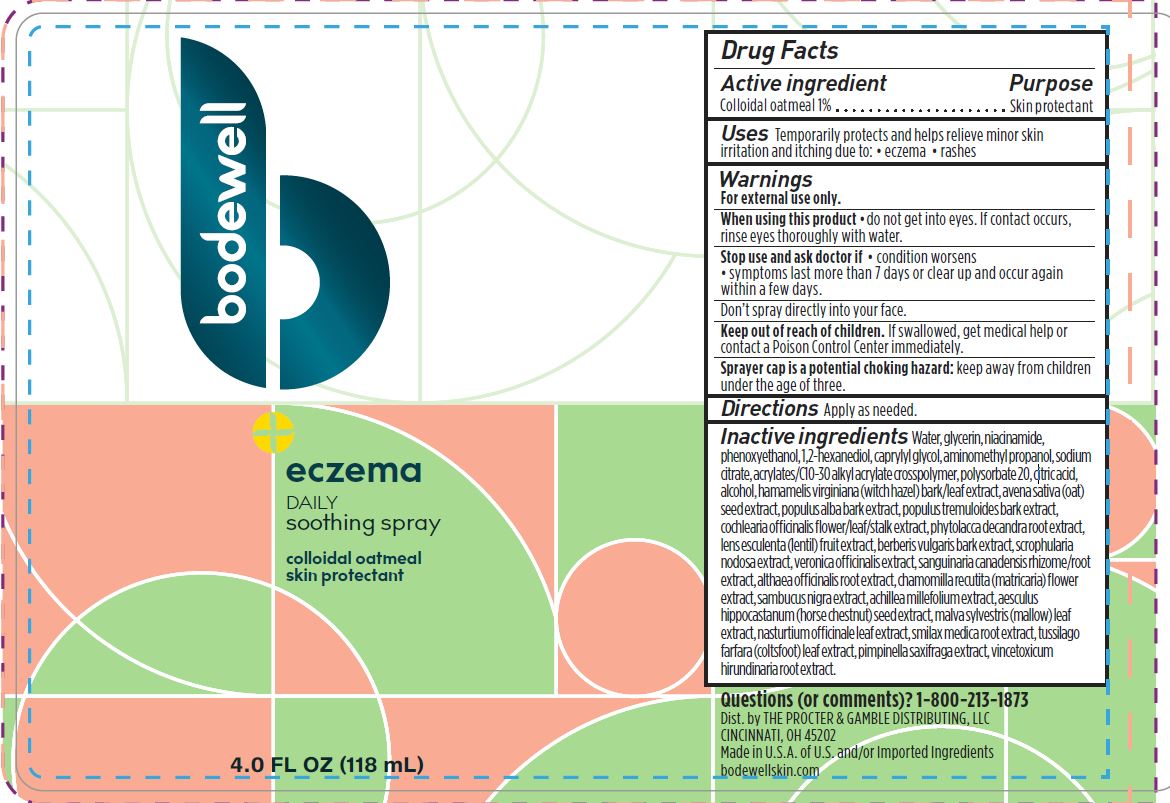
BODEWELL ECZEMA DAILY SOOTHING- colloidal oatmeal spray
The Procter & Gamble Manufacturing Company
----------
Bodewell Eczema Daily Soothing Spray
Warnings
For external use only
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Water, glycerin, niacinamide, phenoxyethanol, 1,2-hexanediol, caprylyl glycol, aminomethyl propanol, sodium citrate, acrylates/C10-30 alkyl acrylate crosspolymer, polysorbate 20, citric acid, alcohol, hamamelis virginiana (witch hazel) bark/leaf extract, avena sativa (oat)
seed extract, populus alba bark extract, populus tremuloides bark extract, cochlearia officinalis flower/leaf/stalk extract, phytolacca decandra root extract, lens esculenta (lentil) fruit extract, berberis vulgaris bark extract, scrophularia nodosa extract, veronica officinalis extract, sanguinaria canadensis rhizome/root extract, althaea officinalis root extract, chamomilla recutita (matricaria) flower extract, sambucus nigra extract, achillea millefolium extract, aesculus hippocastanum (horse chestnut) seed extract, malva sylvestris (mallow) leaf extract, nasturtium officinale leaf extract, smilax medica root extract, tussilago farfara (coltsfoot) leaf extract, pimpinella saxifraga extract, vincetoxicum hirundinaria root extract
| BODEWELL ECZEMA DAILY SOOTHING
colloidal oatmeal spray |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |