HEVERT CALMVALERA COMP.- black cohosh, anamirta cocculus seed, cypripedium parvifolum root, strychnos ignatii seed, passiflora incarnata flowering top, platinum, valerian, and zinc valerate dihydrate injection
Hevert Pharmaceuticals LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
HEVERT® CALMVALERA™ COMP.
| HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Hevert® Calmvalera™ comp. safely and effectively. See full prescribing information for Hevert® Calmvalera™ comp. Hevert® Calmvalera™ comp. solution for injection, for intravenous, intramuscular and subcutaneous administration. Rx Use Only ------------- INDICATIONS AND USAGE ------------- Hevert® Calmvalera™ comp. is a homeopathic drug indicated for the treatment of nervous disorders such as restlessness and sleep disorders, mild depressive states, and mental exhaustion. (1) --------- DOSAGE AND ADMINISTRATION ---------
| -------- DOSAGE FORMS AND STRENGTHS --------
|
| FULL PRESCRIBING INFORMATION: CONTENTS*
1 Indications and Usage 2 Dosage and Administration 2.1 General Instructions 2.2 Standard Dosage 2.3 Acute Dosage 2.4 Instruction for Opening Glass Ampule 3 Dosage Forms and Strength 4 Contraindications 5 Warnings and Precautions 6 Adverse Reactions 6.1 Post-marketing Experience 7 Drug Interactions 8 Use in specific Populations 8.1 Pregnancy |
8.2 Labor and Delivery 8.3 Nursing Mothers 8.4 Pediatric Use 8.5 Geriatric Use 10 Overdosage 11 Description 11.1 Ingredients 11.2 Pharmaceutical Form 11.3 Route of Administration 15 References 16 How Supplied / Storage and Handling 16.1 Dosage Forms and Package Sizes 16.2 Storage * Sections or subsections omitted from the full prescribing information are not listed. |
1 Indications and Usage
1.1 Hevert® Calmvalera™ comp. is a homeopathic drug indicated for the treatment of nervous disorders, such as restlessness and sleep disorders, mild depressive states, and mental exhaustion.
2 Dosage and Administration
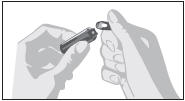
2.1 General Instructions
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Draw up required dose into syringe.
- Discard any unused ampule contents. Do not reuse ampule.
3 Dosage Forms and Strengths
One ampule containing 2 mL solution for injection, containing the active ingredients in the strengths listed under Description.
4 Contraindications
Hevert® Calmvalera™ comp. is contraindicated in patients with known hypersensitivity to lady's slipper or orchids, or to any of its ingredients.
7 Drug Interactions
No interactions have been reported, and none are expected due to the homeopathic dilutions.
8 Use in specific Populations
8.1 Pregnancy
8.1.1 Teratogenic effects
Pregnancy Category C
Animal reproduction studies have not been performed with Hevert® Calmvalera™ comp. or any of its ingredients. There are not adequate and well-controlled studies in pregnant women. Hevert® Calmvalera™ comp. should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
It is not known whether Hevert® Calmvalera™ comp. is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Hevert® Calmvalera™ comp. is administered to a nursing woman.
10 Overdosage
No negative effects of an overdose have been reported and none are expected due to the homeopathic dilutions.
11 Description
11.1 Ingredients
Each 2 mL solution for injection ampule contains:
| Active Ingredients: | |||
|---|---|---|---|
| Ingredient name | Potency | Quantity | Final dilution |
| Cimicifuga racemosa | 4X | 5 % | 5.5X |
| Cocculus indicus | 4X | 5 % | 5.5X |
| Cypripedium pubescens | 4X | 5 % | 5.5X |
| Ignatia amara | 6X | 5 % | 7.5X |
| Passiflora incarnata | 4X | 5 % | 5.5X |
| Platinum metallicum | 10X | 5 % | 11.5X |
| Valeriana officinalis | 4X | 5 % | 5.5X |
| Zincum valerianicum | 8X | 5 % | 9.5X |
Inactive ingredients:
Sodium chloride 0.015 g
16 How Supplied/Storage and Handling
| HEVERT CALMVALERA COMP.
black cohosh, anamirta cocculus seed, cypripedium parvifolum root, strychnos ignatii seed, passiflora incarnata flowering top, platinum, valerian, and zinc valerate dihydrate injection |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Hevert Pharmaceuticals LLC (078647622) |
| Registrant - Hevert Arzneimittel GmbH & Co. KG (318100617) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hevert Arzneimittel GmbH & Co. KG | 318100617 | API MANUFACTURE(54532-0036) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Solupharm Pharmazeutische Erzeugnisse GmbH | 316875129 | MANUFACTURE(54532-0036) | |