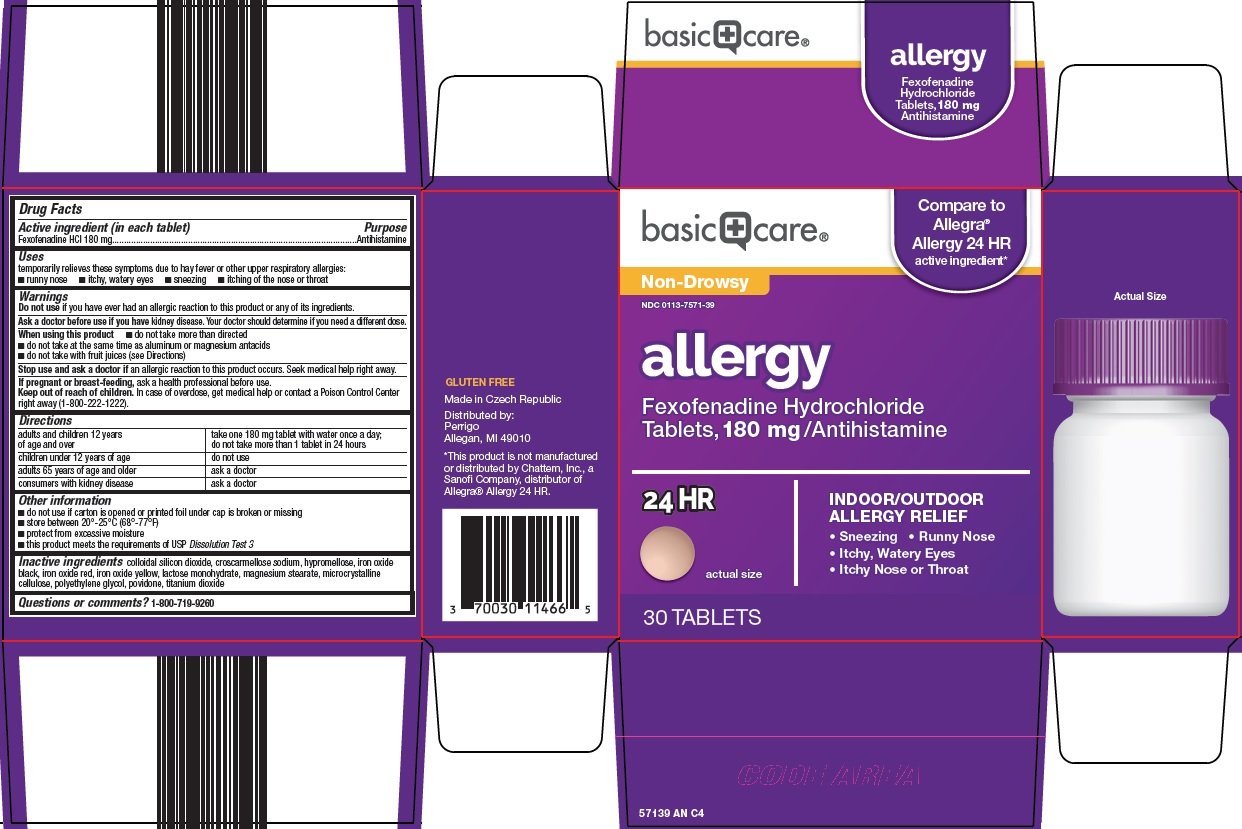
BASIC CARE ALLERGY- fexofenadine hcl tablet, film coated
L. Perrigo Company
----------
Amazon Allergy Drug Facts
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- itchy, watery eyes
- •
- sneezing
- •
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- •
- do not take more than directed
- •
- do not take at the same time as aluminum or magnesium antacids
- •
- do not take with fruit juices (see Directions)
Directions
|
adults and children 12 years of age and over |
take one 180 mg tablet with water once a day; do not take more than 1 tablet in 24 hours |
|
children under 12 years of age |
do not use |
|
adults 65 years of age and older |
ask a doctor |
|
consumers with kidney disease |
ask a doctor |
Other information
- •
- do not use if carton is opened or printed foil under cap is broken or missing
- •
- store between 20°-25°C (68°-77°F)
- •
- protect from excessive moisture
- •
- this product meets the requirements of USP Dissolution Test 3
| BASIC CARE ALLERGY
fexofenadine hcl tablet, film coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - L. Perrigo Company (006013346) |
Revised: 1/2024
Document Id: a267ba00-2b33-495e-8378-758f457ebb17
Set id: d88bc665-8a83-4e91-98cd-844d7e056e8f
Version: 4
Effective Time: 20240112
L. Perrigo Company