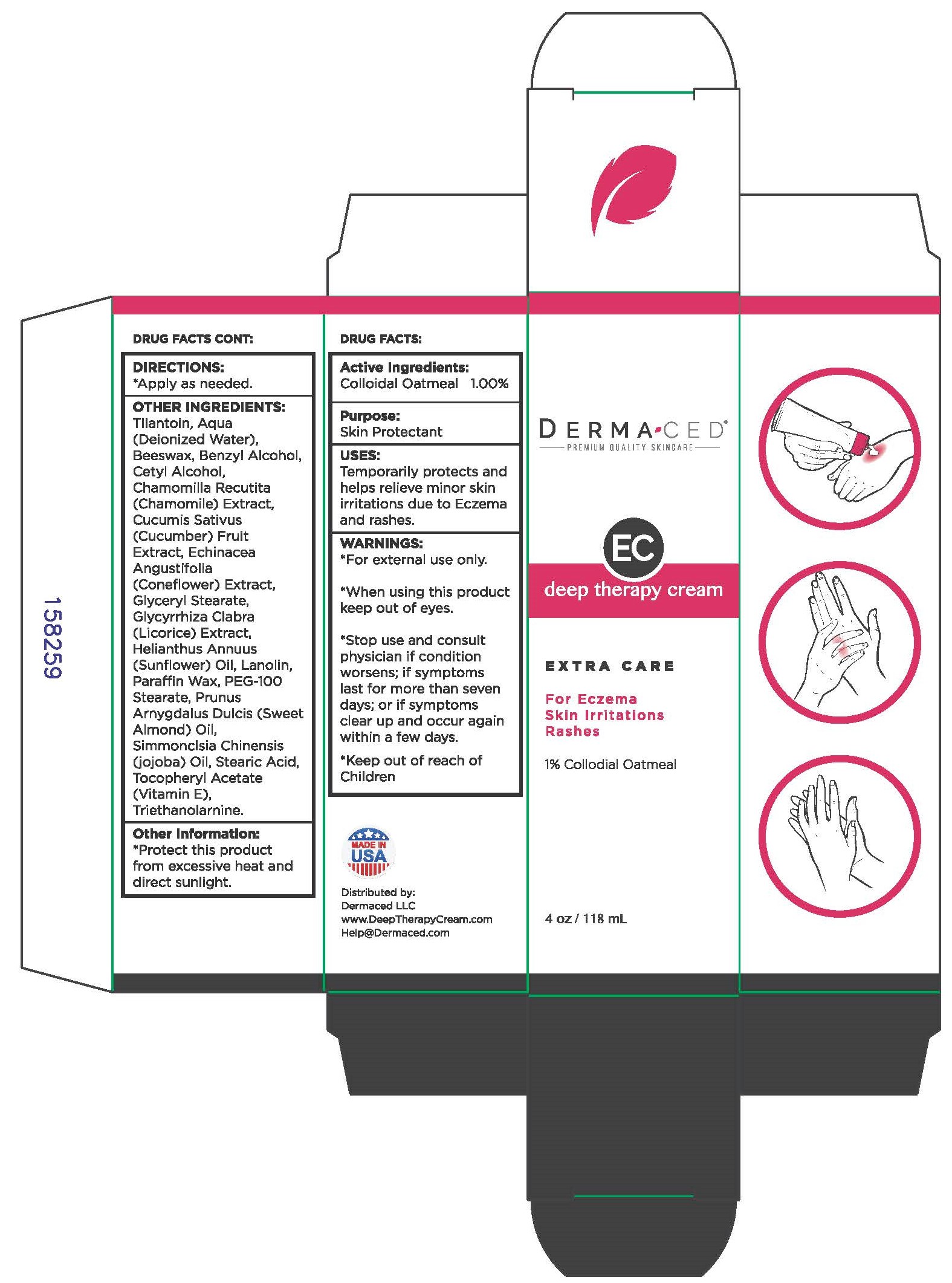
DERMACED DEEP THERAPY ECZEMA- oatmeal cream
Dermaced LLC
----------
DERMACED Deep Therapy Eczema Cream
WARNINGS:
*For external use only.
*When using this product keep out of eyes.
*Stop use and consult physician if condition worsens; if symptoms last for more than seven days; or if symptoms clear up and occur again within a few days.
OTHER INGREDIENTS:
Tllantoin, Aqua (Deionized Water), Beeswax, Benzyl Alcohol, Cetyl Alcohol, Chamomilla Recutita (Chamomile) Extract, Cucumis Sativus (Cucumber) Fruit Extract, Echinacea Angustifolia (Coneflower) Extract, Glyceryl Stearate, Glycyrrhiza Clabra (Licorice) Extract, Helianthus Annuus (Sunflower) Oil, Lanolin, Paraffin Wax, PEG-100 Stearate, Prunus Arnygdalus Dulcis (Sweet Almond) Oil, Simmonclsia Chinensis (jojoba) Oil, Stearic Acid, Tocopheryl Acetate (Vitamin E), Triethanolamine.
| DERMACED DEEP THERAPY ECZEMA
oatmeal cream |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Dermaced LLC (080703517) |