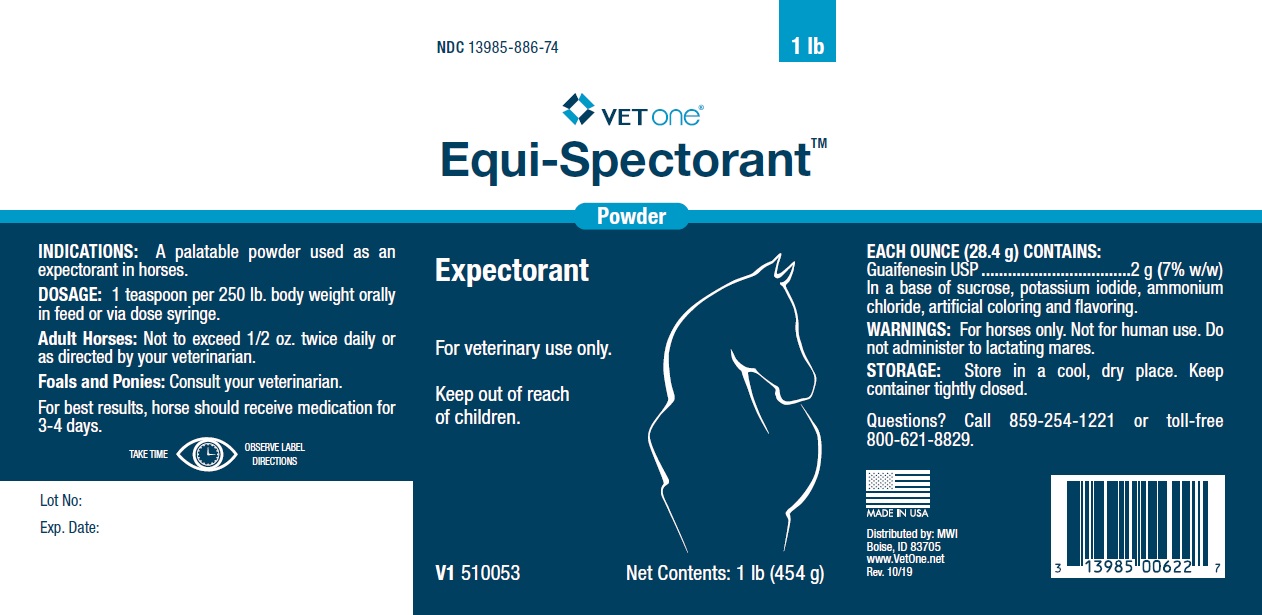
EQUI-SPECTORANT- guaifenesin powder
MWI/VetOne
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Each ounce (28.4g) contains:
Guaifenesin USP..............................2 g (7% w/w)
In a base of sucrose, potassium iodide, ammonium chloride, artificial coloring and flavoring.
| EQUI-SPECTORANT
guaifenesin powder |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - MWI/VetOne (019926120) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Neogen Corporation-Mercer | 042125879 | analysis, manufacture, label | |
Revised: 5/2020
Document Id: 172f08c7-7e12-4199-908f-6fea1391b1cd
Set id: d7198df6-92f2-460a-a0d3-0cbdf4f365f0
Version: 3
Effective Time: 20200502
MWI/VetOne