Label: CLOTRIMAZOLE solution
- NDC Code(s): 10135-671-81, 10135-671-91
- Packager: Marlex Pharmaceuticals Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 6, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
CLOTRIMAZOLE -clotrimazolesolutionMarlexPharmaceuticals, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ClotrimazoleTopical Solution USP,1%
Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- This product is not effective on the scalp or nails For best results, follow directions and continue treatment for length of time indicated. For athlete's foot and ringworm: use daily for 4 weeks. For jock itch: use daily for 2 weeks.
- clean the affected area and dry thoroughly
- apply a thin layer of the product over affected area twice daily (morning and night) or as directed by a doctor
- supervise children in the use of this product
For athlete's foot: pay special attention to spaces between the toes; wear well-fitting, ventilated shoes; change shoes and socks at least once daily
- This product is not effective on the scalp or nails For best results, follow directions and continue treatment for length of time indicated. For athlete's foot and ringworm: use daily for 4 weeks. For jock itch: use daily for 2 weeks.
- Other information
- Inactive ingredient
-
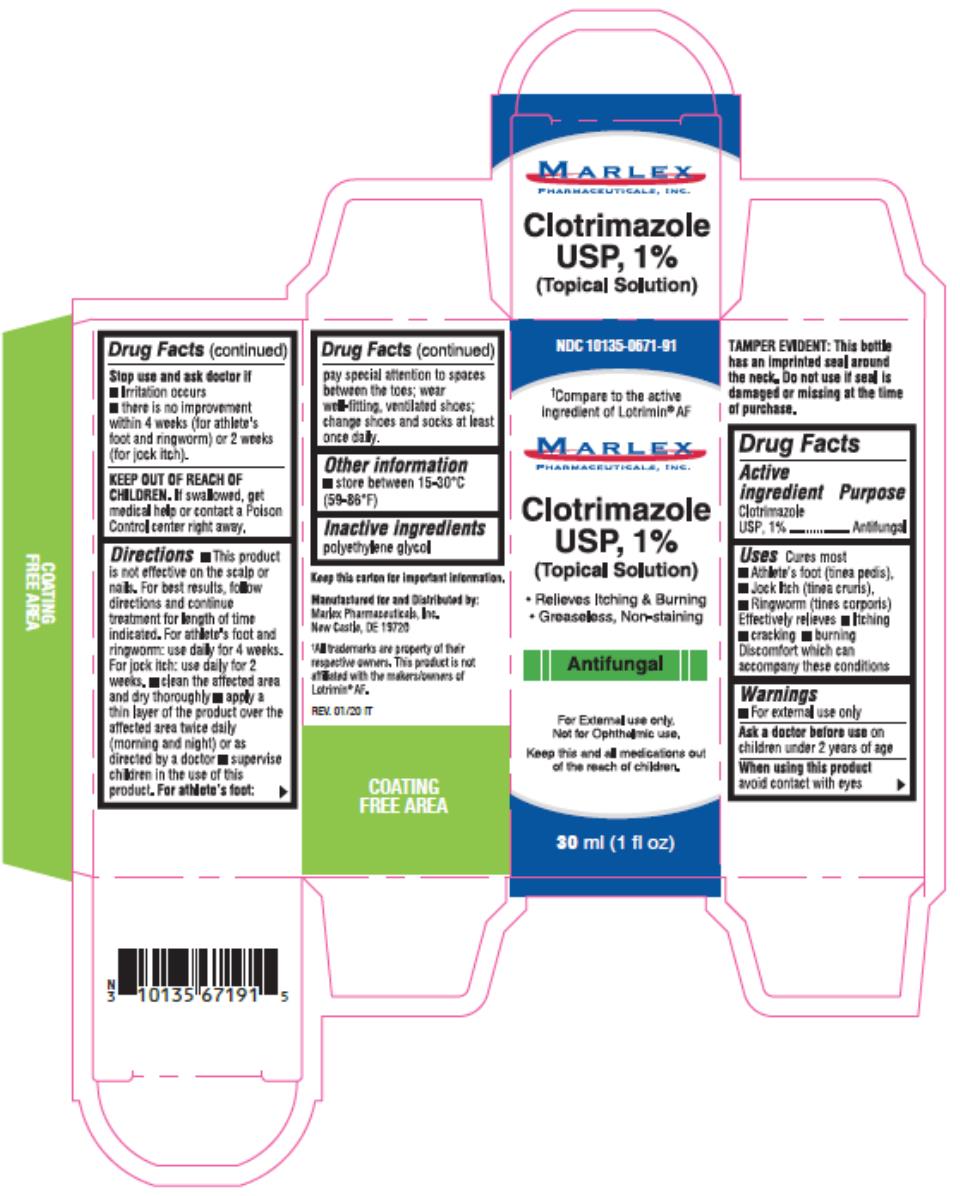
PRINCIPAL DISPLAY PANEL - 10 mL Bottle Carton
NDC 10135-0671-81
Compare to the active ingredient of Lotrimin ®AF*
ClotrimazoleTopical Solution USP,1%
Antifungal
- Relieves Itching & Burning
- Greaseless,Nonstaining
For External use only.
Not for Opthalmic use.
Keep this and all medications out of the reach of children .
MarlexPharmaceuticals, Inc.
10mL

- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLOTRIMAZOLE
clotrimazole solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10135-671 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOTRIMAZOLE (UNII: G07GZ97H65) (CLOTRIMAZOLE - UNII:G07GZ97H65) CLOTRIMAZOLE 1 g in 1 mL Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10135-671-81 1 in 1 CARTON 12/01/2018 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:10135-671-91 1 in 1 CARTON 02/01/2020 2 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 12/01/2018 Labeler - Marlex Pharmaceuticals Inc (782540215)