CPDA-1 - anticoagulant citrate phosphate dextrose adenine solution
Fenwal, Inc.
----------
Instructions for Blood Collection Using (CPDA-1) BLOOD-PACK™ Unit
Rx Only
Integral Donor Tube (IDT)
Use aseptic technique.
Caution: Do not use unless the solutions are clear.
- 1.
- Identify BLOOD-PACK™ unit using appropriate donor identification system.
- 2.
- Adjust donor scale to desired collection weight/volume.
- 3.
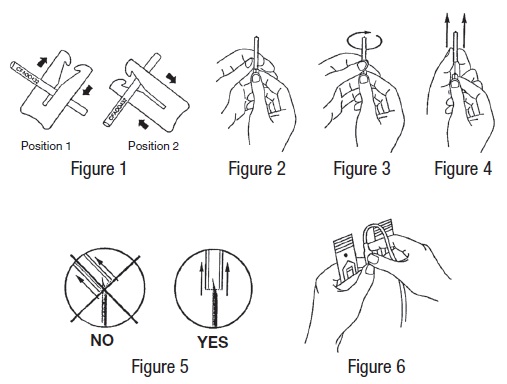
- Suspend primary container from donor scale as far as possible below donor arm and clamp donor tubing at Position 1 (see Figure 1). Use one of the clamps that come inside the aluminum pouch.
- 4.
- Apply pressure to donor’s arm and disinfect site of venipuncture.
- 5.
- If blood pressure cuff is used, inflate to approximately 60 mm Hg.
- 6.
- Remove needle cover per instructions below:
- a)
- Hold the needle hub upwards. With the other hand, grasp the base of the needle cover (Figure 2), twist approximately 1/4 turn to break tamper evident seal (Figure 3).
- b)
- Remove needle cover (Figure 4), be careful not to drag cover across the needle point (Figure 5).
- 7.
- Perform venipuncture, appropriately secure donor needle and/or tubing and move clamp to Position 2 (Figure 1).
- 8.
- Mix blood and anticoagulant at several intervals during collection and immediately after collection.
- 9.
- Collect labeled volume of blood. Anticoagulant volume is sufficient for labeled volume ± 10%.
- 10.
- Place clamp back to Position 1 (Figure 1).
- 11.
- Seal donor tubing between clamp and primary container (a hand sealer clamp may be used). Dispose of needle and tubing appropriately. Cut between clamp and clip/seal; collect any needed samples using standard techniques.
- 12.
- Release pressure on donor’s arm and withdraw needle.
- 13.
- Strip blood from donor tubing into container, mix and allow the tubing to refill; repeat once. Seal at X marks on donor tubing to provide numbered aliquots of anticoagulated blood for typing or crossmatching.
- 14.
- Store suspended Red Blood Cells between 1 and 6°C.
- 15.
- Infuse suspended Red Blood Cells within 35 days of collection.
NOTE:
In order to establish fluid transfer from primary container to secondary system, hold the cannula by its base with one hand, and with the other hand break the cannula by bending it 90º in one direction, then 180º in the opposite direction (Figure 6).
For further processing, use standard component processing and storage techniques.
Dispose of containers and materials into appropriate biohazardous waste containers following established procedures.
Sterile, non-pyrogenic fluid path. Steam sterilized. Single use only.
Store at Controlled Room Temperature.
USP Definition of “Controlled Room Temperature”
United States Pharmacopeia, General Notices.
United States Pharmacopeial Convention, Inc.
12601 Twinbrook Parkway, Rockville, MD
 Manufacturer
Manufacturer
 Manufactured by:
Manufactured by:
Fenwal International, Inc.
Road 357, Km. 0.8
Maricao, PR 00606
Made in USA
Imported and distributed in India by:
Fenwal India Pvt Ltd
Upper Ground Floor, Tower B
DLF Building No. 10, DLF Cyber City
DLF Phase-II, Gurgaon 122 002,
Haryana, India
Import License No.: FF-504-14890
Imported and distributed in Indonesia by:
PT.Medquest Jaya Global
Menara Salemba 6th Floor
Jl.Salemba Raya Kav 5-5A
Jakarta-Indonesia 10440
Reg. No.: DEPKES RI AKL 20209902358
Imported and distributed in Thailand by:
Fenwal (Thailand) Ltd.
17th Fl. Thanapoom Tower,
1550 New Petchburi Rd., Makasan
Rajthevi, Bangkok 10400
Thailand
Imported and distributed in Venezuela by:
SOLUCARE GT, C.A. RIF J-30512494-9
en La Urbina, Caracas,
Republica Bolivariana de Venezuela
TELF (0212) 2436663
Registrado en el MPPS bajo el No:
07-19-07-227 REV: A 05/2012
FENWAL and BLOOD-PACK are trademarks of Fenwal, Inc.
© 2012 Fenwal, Inc. All rights reserved.
PACKAGE/LABEL DISPLAY PANEL
Code T4R1339UT
6 Units
Fenwal™
Triple BLOOD-PACK™ Unit Anticoagulant Citrate Phosphate Dextrose Adenine Solution (CPDA-1)
Rx only
Triple BLOOD-PACK™ unit for collection of 350 mL of blood, 16 gauge needle.
Each unit consists of a primary container with 49 mL CPDA-1 solution containing 1.56 g Dextrose (monohydrate), USP, 1.29 g Sodium Citrate (dihydrate), USP, 160 mg Citric Acid (monohydrate), USP, 109 mg Monobasic Sodium Phosphate (monohydrate), USP, 13.5 mg Adenine, USP, Water for Injection USP to 49 mL, and two empty 400 mL TRANSFER PACK™ containers.
See instructions for use. Sterile, non-pyrogenic fluid path. Steam sterilized. Single use only. Do not vent. Do not use if there is any visible sign of deterioration. Dispose of container appropriately.
Store at Controlled Room Temperature (refer to direction insert).
- •
- Unused units in open foil pouch may be kept to 60 days by folding and securing open end of foil pouch to prevent possible loss of moisture.
- •
- Direct handling of product surfaces prior to extended storage in the foil pouch, may result in mold growth.
- •
- Units removed from the foil pouch must be used within 4 days (96 hours). Units out of the foil pouch for longer than 4 days must be discarded.
 Manufactured by:
Manufactured by:
Fenwal International, Inc.
Road 357, Km. 0.8
Maricao, PR 00606
Made in USA
07-28-04-648 REV: A
Imported and distributed in Thailand by:
Fenwal (Thailand) Ltd.
17th Fl. Thanapoom Tower
1550 New Petchburi Rd., Makasan
Rajthevi, Bangkok 10400
Thailand
Reg. No.: See Instructions for use.
Imported and distributed in Indonesia by:
PT. Medquest Jaya Global
Menara Salemba 6th Floor
Jl.Salemba Raya Kav 5-5A
Jakarta-Indonesia 10440
Reg. No.: DEPKES RI AKL 20209902358
FENWAL, BLOOD-PACK, and TRANSFER PACK are trademarks of Fenwal, Inc.

| CPDA-1
anticoagulant citrate phosphate dextrose adenine solution |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Fenwal, Inc. (794519020) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Fenwal International, Inc. | 091164590 | MANUFACTURE(0942-6332) | |