IMPAVIDO- miltefosine capsule
Paladin Therapeutics Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use IMPAVIDO safely and effectively. See full prescribing information for IMPAVIDO.
IMPAVIDO (miltefosine) capsules, for oral use Initial U.S. Approval: 2014 WARNING: EMBRYO-FETAL TOXICITYSee full prescribing information for complete boxed warning.IMPAVIDO may cause fetal harm. Fetal death and teratogenicity, occurred in animals administered miltefosine at doses lower than the recommended human dose. Do not administer IMPAVIDO to pregnant women. Obtain a serum or urine pregnancy test in females of reproductive potential prior to prescribing IMPAVIDO. Advise females of reproductive potential to use effective contraception during therapy and for 5 months after therapy (4.1, 5.1, 8.1, 8.8, 13.1). INDICATIONS AND USAGEIMPAVIDO is an antileishmanial drug indicated in adults and adolescents ≥12 years of age weighing ≥30 kg (66 lbs) for treatment of:
Limitations of use: Leishmania species evaluated in clinical trials were based on epidemiologic data. There may be geographic variation in the response of the same Leishmania species to IMPAVIDO (1, 14). The efficacy of IMPAVIDO in the treatment of other Leishmania species has not been evaluated. DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSEach IMPAVIDO capsule for oral use contains 50 mg miltefosine (3). CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Paladin Therapeutics Inc. at 1-888-550-6060 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSUSE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 5/2018 |
FULL PRESCRIBING INFORMATION
WARNING: EMBRYO-FETAL TOXICITY
IMPAVIDO may cause fetal harm. Fetal death and teratogenicity occurred in animals administered miltefosine at doses lower than the recommended human dose. Do not administer IMPAVIDO to pregnant women. Obtain a serum or urine pregnancy test in females of reproductive potential prior to prescribing IMPAVIDO. Females of reproductive potential should be advised to use effective contraception during IMPAVIDO therapy and for 5 months after therapy [see Contraindications (4.1), Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.8) and Nonclinical Toxicology (13.1)].
1 INDICATIONS AND USAGE
IMPAVIDO (miltefosine) capsules are indicated in adults and adolescents ≥12 years of age weighing ≥ 30 kg for the treatment of:
- •
- Visceral leishmaniasis caused by Leishmania donovani [see Clinical Trials (14.1)].
- •
- Cutaneous leishmaniasis caused by Leishmania braziliensis, Leishmania guyanensis, and Leishmania panamensis [see Clinical Trials (14.2)].
- •
- Mucosal leishmaniasis caused by Leishmania braziliensis [see Clinical Trials (14.3)].
Limitations of Use:
- •
- Leishmania species studied in clinical trials evaluating IMPAVIDO were based on epidemiologic data [see Clinical Trials (14.1, 14.2)].
- •
- There may be geographic variation in clinical response of the same Leishmania species to IMPAVIDO [see Clinical Trials (14.1, 14.2)].
- •
- The efficacy of IMPAVIDO in the treatment of other Leishmania species has not been evaluated.
2 DOSAGE AND ADMINISTRATION
The treatment duration is 28 consecutive days. Administer with food to ameliorate gastrointestinal adverse reactions.
|
Weight |
Dosage and Administration |
|
30 kg to 44 kg |
One 50 mg capsule twice daily with food (breakfast and dinner) |
|
45 kg or greater |
One 50 mg capsule three times daily with food (breakfast, lunch, and dinner) |
3 DOSAGE FORMS AND STRENGTHS
IMPAVIDO® (miltefosine) oral capsules are opaque, red, hard gelatin capsules with “PLB” imprinted on the capsule body and “MILT 50” imprinted on the cap using a white ink. Each capsule contains 50 mg miltefosine [see Description (11), How Supplied/Storage and Handling (16)].
4 CONTRAINDICATIONS
4.1 Pregnancy
IMPAVIDO may cause fetal harm. IMPAVIDO is contraindicated in pregnant women. Obtain a urine or serum pregnancy test prior to prescribing IMPAVIDO [see Boxed Warning and Use in Specific Populations (8.1)].
4.2 Sjögren-Larsson-Syndrome
IMPAVIDO is contraindicated in patients who have Sjögren-Larsson-Syndrome [see Clinical Pharmacology (12.3)].
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
Miltefosine may cause fetal harm. Embryo-fetal toxicity, including death and teratogenicity, was observed in animals administered miltefosine prior to mating, during early pregnancy, and during organogenesis at doses lower than the maximum recommended human dose (MRHD). Do not use IMPAVIDO in pregnant women. Obtain a urine or serum pregnancy test prior to prescribing IMPAVIDO to females of reproductive potential. Advise females of reproductive potential to use effective contraception during IMPAVIDO therapy and for 5 months after completion of therapy [see Boxed Warning, Contraindications (4.1) and Use in Specific Populations (8.1, 8.8)].
5.2 Reproductive Effects
Females
Miltefosine caused impaired fertility in rats and reversible follicular atresia and diestrus in dogs at doses approximately 1.0 and 0.2 times respectively the MRHD based on body surface area comparisons [see Nonclinical Toxicology (13.1)]. Effects on human female fertility have not been formally studied.
Males
Miltefosine caused reduced viable sperm counts and impaired fertility in rats at doses approximately 0.4 times the MRHD [see Nonclinical Toxicology (13.1)]. A higher dose in rats, approximately 1.0 times the MRHD, caused testicular atrophy and impaired fertility that did not fully reverse 10 weeks after drug administration ended.
Scrotal pain and decreased or absent ejaculation during therapy have been reported during IMPAVIDO therapy [see Adverse Reactions (6.2)]. The effects of IMPAVIDO on human male fertility have not been adequately studied.
Advise women and men of the animal fertility findings, and that the potential for impaired fertility with IMPAVIDO therapy in humans has not been adequately evaluated.
5.3 Renal Effects
Elevations of serum creatinine (Cr) were noted in clinical trials evaluating IMPAVIDO in the treatment of cutaneous, mucosal and visceral leishmaniasis. Monitor renal function weekly in patients receiving IMPAVIDO during therapy and for 4 weeks after end of therapy [see Adverse Reactions (6.1)].
5.4 Hepatic Effects
Elevations in liver transaminases (ALT, AST) and bilirubin were noted in clinical trials evaluating IMPAVIDO in the treatment of visceral leishmaniasis. Monitor liver transaminases (ALT, AST) and bilirubin during therapy in patients receiving IMPAVIDO [see Adverse Reactions (6.1)].
5.5 Gastrointestinal Effects
Vomiting and/or diarrhea commonly occur during IMPAVIDO administration and may result in volume depletion. Encourage fluid intake to avoid volume depletion [see Adverse Reactions (6.1)].
5.6 Thrombocytopenia
Thrombocytopenia during therapy has been reported in patients treated for visceral leishmaniasis. Monitor platelet count during therapy for visceral leishmaniasis [see Adverse Reactions (6.1, 6.2)].
5.7 Absorption of Oral Contraceptives
Vomiting and/or diarrhea occurring during IMPAVIDO therapy may affect the absorption of oral contraceptives, and therefore compromise their efficacy. If vomiting and/or diarrhea occur during IMPAVIDO therapy, advise females to use additional non-hormonal or alternative method(s) of effective contraception.
5.8 Stevens-Johnson Syndrome
Stevens-Johnson syndrome has been reported during IMPAVIDO therapy. Discontinue IMPAVIDO if an exfoliative or bullous rash is noted during therapy [see Adverse Reactions (6.1)].
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Clinical Trials Experience
Visceral Leishmaniasis
One Phase 3 trial was conducted in patients ≥ 12 years of age in India. Two-hundred and ninety-nine (299) patients (211 men and 88 women) received oral IMPAVIDO at a target dose of 2.5 mg/kg/day for 28 days (50 mg capsule once daily if weight was less than 25 kg and 50 mg capsule twice daily if weight was 25 kg or greater). Patients ranged between 12 and 64 years of age. Weight ranged between 15 and 67 kg (mean weight 38.6 kg) and BMI ranged between 8.2 and 24 (mean 16.1). Ninety-nine (99) patients received 1 mg/kg/day amphotericin B deoxycholate intravenously every other day for 15 doses. A statistically significant higher percentage of men received IMPAVIDO compared to amphotericin B.
Less than 1% of patients who received IMPAVIDO died (2/299) and no patient who received amphotericin B died. Serious adverse reactions were reported in 2% of IMPAVIDO recipients (6/299) and 1% of amphotericin B recipients (1/99). Approximately 3% of patients discontinued treatment in each treatment arm due to an adverse reaction. Serious adverse reactions and adverse reactions leading to drug discontinuation that were thought to be related or possibly related to IMPAVIDO included Stevens-Johnson syndrome, melena and thrombocytopenia, arthritis and skin rash, CTCAE1 Grade 4 diarrhea (≥10 stools per day) and CTCAE Grade 4 hyperbilirubinemia (≥10x upper limit of normal ULN).
|
System Organ Class |
IMPAVIDO |
Amphotericin B Deoxycholate |
|
Gastrointestinal Disorders | ||
|
Diarrhea |
61 (20.4%) |
6 (6.1%) |
|
Vomiting |
113 (37.8%) |
20 (20.0%) |
|
General Disorders | ||
|
Asthenia |
19 (6.3%) |
4 (4.0%) |
|
Metabolism and Nutrition Disorders | ||
|
Decreased Appetite |
69 (23.1%) |
22 (22.2%) |
In this study, creatinine (Cr) elevations ≥ 1.5 times above baseline occurred in approximately 10% of IMPAVIDO recipients and in 40% of amphotericin B recipients at the end of therapy. Ten percent of subjects in each arm had Cr elevations ≥1.5 times above baseline at 6 months follow up. No IMPAVIDO recipient discontinued therapy due to Cr elevation.
Elevations of transaminases during therapy occurred in up to half of IMPAVIDO recipients and up to a third of amphotericin B recipients. The elevations were mild (< 3x ULN) or moderate (3-5x ULN) in 94% and 6% respectively of IMPAVIDO-treated patients who experienced an elevation. No patient discontinued therapy due to elevations in transaminases.
At the end of therapy, 62% and 2.4% of IMPAVIDO recipients and 54% and 2% of amphotericin B recipients had platelet count < 150,000 and < 50,000 respectively.
Cutaneous Leishmaniasis
The efficacy of IMPAVIDO in the treatment of cutaneous leishmaniasis was evaluated in one placebo-controlled trial conducted in Colombia and Guatemala and in two comparative trials conducted in Bolivia and Brazil respectively. In the placebo-controlled trial, eighty-nine (89) patients ≥12 years of age received a target IMPAVIDO dose of 2.5 mg/kg/day for 28 days and forty-four (44) received placebo. In the comparative trials, one hundred and twenty (120) patients ≥12 years of age received a target IMPAVIDO dose of 2.5 mg/kg/day for 28 days and fifty eight (58) patients received 20 mg/kg/day pentavalent antimony (meglumine) parenterally for 20 days.
|
System Organ Class |
IMPAVIDO |
Placebo |
|
Ear and Labyrinth Disorders | ||
|
Motion Sickness |
26 (29.2%) |
10 (22.7%) |
|
Gastrointestinal Disorders | ||
|
Abdominal Pain |
10 (11.2%) |
3 (6.8%) |
|
Diarrhea |
7 (7.9%) |
2 (4.5%) |
|
Nausea |
32 (35.9%) |
5 (11.1%) |
|
Vomiting |
4 (4.5%) |
0 |
|
General and Administration Site Disorders | ||
|
Malaise |
3 (3.4%) |
1 (2.3%) |
|
Pyrexia |
5 (5.6%) |
2 (4.5%) |
|
Nervous System Disorders | ||
|
Dizziness |
4 (4.5%) |
0 |
|
Headache |
25 (28.1%) |
10 (22.7%) |
|
Somnolence |
3 (3.4%) |
0 |
|
Skin and Subcutaneous Tissue Disorders | ||
|
Pruritus |
4 (4.5%) |
0 |
|
System Organ Class |
IMPAVIDO |
Meglumine |
|
Gastrointestinal Disorders | ||
|
Abdominal Pain |
9 (7.5%) |
3 (5.2%) |
|
Diarrhea |
18 (15.0%) |
3 (5.2%) |
|
Nausea |
50 (41.7%) |
3 (5.2%) |
|
Vomiting |
33 (27.5%) |
0 |
|
Infections and Infestations | ||
|
Lymphangitis |
7 (5.8%) |
0 |
|
Metabolism and Nutrition Disorders | ||
|
Decreased Appetite |
13 (10.8%) |
4 (5.8%) |
|
Nervous System Disorders | ||
|
Dizziness |
15 (12.5%) |
4 (6.9%) |
|
Skin and Subcutaneous Tissue Disorders | ||
|
Pruritus |
7 (5.8%) |
0 |
In the placebo controlled trial, 12/89 (13.4%) IMPAVIDO subjects had Cr increases of 1.5-3 times above baseline, compared to 2/44 (4.5%) placebo subjects at end of therapy. In the comparative trial, a similar percentage of subjects who received IMPAVIDO or pentavalent antimony had Cr elevations above baseline at 3 and 6 months after therapy (approximately 5%). Approximately 25% of IMPAVIDO subjects and 11% of pentavalent antimony subjects had Cr elevations 1.5-3 times above baseline at the end of therapy in the two active controlled trials. The frequency of AST and ALT increase above upper limit of normal at end of therapy was similar in IMPAVIDO and placebo recipients (approximately 5%).
Other adverse events seen at <2% incidence in the IMPAVIDO group included anemia, lymphadenopathy, abdominal distension, constipation, dysphagia, flatulence, fatigue, malaise, abscess, cellulitis, ecthyma, paresthesia, testicular pain, testicular swelling, Stevens-Johnson syndrome, urticaria, rash, pyoderma.
- 1
- Common Terminology Criteria for Adverse Events
6.2 Postmarketing Experience
The following adverse reactions have been identified during use of IMPAVIDO worldwide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatics Disorders: thrombocytopenia, agranulocytosis
Gastrointestinal Disorders: melena
General Disorders: generalized edema, peripheral edema
Hepatobiliary Disorders: jaundice
Nervous System Disorders: seizure
Reproductive System and Breast Disorders: scrotal pain, decreased ejaculate volume, absent ejaculation.
Vascular Disorders: epistaxis
7 DRUG INTERACTIONS
In vitro and animal metabolism studies showed that miltefosine did not markedly induce or inhibit the activity of the major human cytochrome P450 enzymes [see Clinical Pharmacology (12.3)]. The potential of miltefosine to interact with drug transporters has not been evaluated.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D
Risk Summary
IMPAVIDO may cause fetal harm. Human pregnancy data are not available, however, embryo-fetal toxicity including death and teratogenicity, was observed in embryo-fetal studies in rats and rabbits administered oral miltefosine during organogenesis at doses that were respectively 0.06 and 0.2 times the maximum recommended human dose (MRHD), based on body surface area (BSA) comparison. Numerous visceral and skeletal fetal malformations were observed in a fertility study in female rats administered miltefosine prior to mating through day 7 of pregnancy at doses 0.3 times the MRHD. Do not administer IMPAVIDO to pregnant women.
Clinical Considerations
During pregnancy, visceral leishmaniasis may be life-threatening for the mother and may result in adverse fetal outcomes, including spontaneous abortion, congenital disease due to vertical transmission, small for gestational age newborn, and severe anemia. During pregnancy, cutaneous leishmaniasis may manifest with larger and atypical appearing lesions and may be associated with increased risk for adverse fetal outcomes, including preterm births and stillbirths.
Animal Data
Miltefosine administration in rat embryo-fetal toxicity studies during early embryonic development (Day 6 to Day 15 of gestation) caused embryo-fetal toxicity including death and teratogenicity at dosages of ≥ 1.2 mg/kg/day (0.06 times the MRHD based on BSA comparison). Teratogenic effects included undeveloped cerebrum, hemorrhagic fluid filling the lumina of the skull, cleft palate and generalized edema. Embryo-fetal toxicity was also observed in rabbits after oral administration of miltefosine during organogenesis (Day 6 to Day 18 of gestation) at doses ≥ 2.4 mg/kg/day (0.2 times the MRHD based on BSA comparison). In both rats and rabbits, there were no viable litters at miltefosine doses ≥ 6.0 mg/kg/day (0.3 or 0.6 times the MRHD based on BSA comparisons for rats and rabbits respectively).
In a separate female fertility study in rats, miltefosine doses ≥ 6.81 mg/kg/day (0.3 times the MRHD based on BSA comparison) administered for four weeks before mating and up to Day 7 of pregnancy produced numerous visceral (misshapen cerebral structures, dilated ventricles filled with brown masses, misshapen spinal cord, misshapen and malpositioned eyes, hypophysis, and absent inner ear) and skeletal (cleft palate, dumbbell-shaped ossification of thoracic vertebral centers, markedly enlarged skull bones, and markedly dilated suturae) fetal malformations. [see Contraindications (4.1), Nonclinical Toxicology (13.1)].
8.3 Nursing Mothers
It is not known whether IMPAVIDO is present in human milk. Because many drugs are present in human milk and because of the potential for serious adverse reactions in nursing infants from IMPAVIDO, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother. Breastfeeding should be avoided for 5 months after IMPAVIDO therapy.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients < 12 years have not been established. Juvenile rats were more sensitive to the miltefosine-induced effects, especially retinal and kidney effects, than adult rats [see Indications and Usage (1)].
8.5 Geriatric Use
Clinical studies of IMPAVIDO did not include sufficient numbers of subjects 65 years of age and over to determine if they respond differently than younger subjects.
8.6 Renal Impairment
Patients with serum creatinine or BUN levels ≥1.5 times the upper limit of normal were excluded from the clinical studies. Miltefosine pharmacokinetics have not been studied in patients with renal impairment.
8.7 Hepatic Impairment
Patients with serum levels of ALT or AST ≥3 times the upper limit of normal and bilirubin levels ≥2 times the upper limit of normal were excluded from the clinical studies. Miltefosine pharmacokinetics have not been studied in patients with hepatic impairment.
8.8 Females and Males of Reproductive Potential
Contraception
IMPAVIDO may cause fetal harm when used during pregnancy. Advise females of reproductive potential to use effective contraception during IMPAVIDO therapy and for 5 months after therapy is completed [see Boxed Warning, Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
Vomiting and/or diarrhea occurring during IMPAVIDO therapy may affect absorption of oral contraceptives and therefore may compromise their efficacy. Advise females who use oral contraceptives to use additional non-hormonal or alternative method(s) of effective contraception during IMPAVIDO therapy if vomiting and/or diarrhea occurs during therapy [see Warnings and Precautions (5.7)].
Infertility
Females
Miltefosine caused impaired fertility in rats and caused reversible follicular atresia and diestrus in dogs at doses approximately 1.0 and 0.2 times respectively the MRHD [see Warnings and Precautions (5.2), Nonclinical Toxicology (13.1)]. The effects of IMPAVIDO on human female fertility have not been formally studied.
Males
Miltefosine caused reduced viable sperm counts and impaired fertility in rats at doses approximately 0.4 times the MRHD [see Warnings and Precautions (5.2), Nonclinical Toxicology (13.1)]. A higher dose in rats, approximately 1.0 times the MRHD, caused testicular atrophy and impaired fertility that did not fully reverse 10 weeks after drug administration ended. The effects of IMPAVIDO on human male fertility have not been adequately studied.
Advise women and men of the animal fertility findings, and that the potential for impaired fertility with IMPAVIDO therapy has not been adequately evaluated.
10 OVERDOSAGE
The common adverse effects of vomiting, diarrhea, and abdominal pain are likely in case of overdose. Institute adequate hydration to prevent the risk of impaired renal function, and replace electrolytes as necessary. Because miltefosine is only slightly excreted in the urine, forced diuresis will not increase miltefosine excretion. Gastrointestinal lavage is of unknown value. A specific antidote to treat miltefosine overdose is not known.
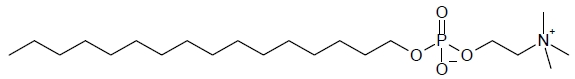
11 DESCRIPTION
IMPAVIDO capsules contain the active ingredient miltefosine, an antileishmanial agent. The chemical name of miltefosine is 2-[[(hexadecyloxy)hydroxyphosphenyl]oxy]-N,N,N-trimethylethylammonium inner salt. Miltefosine is a white powder that is freely soluble in water, 0.1 N HCl or NaOH, methanol, and ethanol. It has the empirical formula of C21H46NO4P with a molecular weight of 407.6 and the following structural formula:
The inactive ingredients are colloidal silicon dioxide, microcrystalline cellulose, lactose monohydrate, talc, and magnesium stearate. The capsule shell contains gelatin, titanium dioxide, ferric oxide, and purified water.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Miltefosine is an anti-leishmanial agent [see Clinical Pharmacology (12.4)].
12.3 Pharmacokinetics
The pharmacokinetic parameters of miltefosine in patients with visceral and cutaneous leishmaniasis treated for 28 days with IMPAVIDO are listed in Table 5. Due to the long half-life of miltefosine (> 6 days), trough plasma concentrations did not appear to reach a steady state at the end of treatment (i.e., Day 28).
| a: Adolescent (≥12 years)/Adults, mean dose per kg was 3.1 mg/kg/day b: Adolescent (≥12 years)/Adults, mean dose per kg was 3.6 mg/kg/day c: Adults, mean dose per kg was 1.8 mg/kg/day d: median (range) e: AUC0-12h for BID, AUC0-8h for TID f: t1/2,α = distribution phase half-life; t1/2,β = terminal elimination phase half-life g: Estimates based on a population PK model h: mean (% standard error) |
||||||
|
Dose |
Cmax
|
Tmaxd
|
AUCtaue
|
t1/2,αf
|
t1/2,βg
|
|
|
Visceral Leishmaniasis |
50 mg BID (4 wks)a |
66.2 (28.5) |
7(2-12) |
636 (26.7) |
6.4 (31.1) | |
|
50 mg BID (1 wk)/ |
75.9 (17.6) |
4 (2-8) |
486 (18.1) |
8.5 (28.9) | ||
|
Cutaneous Leishmaniasisc
|
50 mg TID (4 wks) |
37.3 (22)f |
295 (22)f |
6.8 (5.8)g,h |
30.7 (18.3)g,h |
|
Absorption
Absolute bioavailability of miltefosine has not been determined. In patients with visceral leishmaniasis, maximum miltefosine concentrations following oral administration of IMPAVIDO capsules were reached right before the next dose in many patients, indicating that the absorption of miltefosine may proceed throughout the dosing interval.
Distribution
The distribution of miltefosine has not been studied in humans. Human plasma protein binding of miltefosine, evaluated by an ultracentrifugation method, was 98% over the drug concentration range from 0.1 to 10 µg/mL. In rats, radioactivity of [14C] miltefosine is widely distributed after both single and repeated oral administration with highest uptake of radioactivity in kidney, liver, and spleen. Placental transfer and excretion into milk have not been investigated.
Metabolism and Excretion
No in vitro oxidative metabolism by 15 different human cytochrome P450 enzymes (1A1, 1A2, 1B1, 2A6, 2B6, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 3A4, 3A5, 3A7, and 4A1) was observed.
A slow metabolic breakdown could be shown in human hepatocytes, resulting in the release of choline by phospholipase D-like cleavage of the miltefosine molecule. The fatty alcohol-containing fragment of miltefosine can enter the metabolism of fatty acids after being oxidized to palmitic acid. This oxidation is blocked in patients with Sjögren-Larsson syndrome, which is caused by a genetic defect in fatty aldehyde dehydrogenase activity. IMPAVIDO is contraindicated in patients who have Sjögren-Larsson-Syndrome [see Contraindications (4.2)].
There was little or no evidence of time or metabolism dependent inhibition of the cytochrome P450 enzymes examined at up to approximately 40 µg/mL miltefosine.
Oral administration of miltefosine did not markedly induce the content of hepatic CYP3A assayed by demethylation activity of erythromycin in rats.
In visceral leishmaniasis patients, <0.2% of the administered dose was excreted into the urine.
12.4 Microbiology
Mechanism of Action
The specific mode of action of miltefosine against Leishmania species is unknown. The mechanism of action of miltefosine is likely to involve interaction with lipids (phospholipids and sterols), including membrane lipids, inhibition of cytochrome c oxidase (mitochondrial function), and apoptosis-like cell death.
Activity In Vitro and In Vivo
Miltefosine has anti-leishmanial activity in vitro and in clinical infections [see Clinical Studies (14)]. Sensitivity of different Leishmania species as well as different strains of a Leishmania species to miltefosine may vary in different geographic regions.
Drug Resistance
In vitro studies show a potential for development of resistance to miltefosine. Some strains of L. braziliensis with intrinsic resistance to miltefosine have been identified. However, the clinical relevance of these observations is not known.
Drug resistance could be due to a decrease in miltefosine accumulation within Leishmania parasite which is thought to be due to either an increase in drug efflux, mediated by the overexpression of the ABC transporter P-glycoprotein and/or a decrease in drug uptake by the inactivation of the miltefosine transport machinery that consists of the miltefosine transporter and its beta subunit. Mutation in the transporter gene was reported in the isolates from a relapsed patient in one study. However, the clinical relevance of these findings is not known.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenicity/Carcinogenicity: Miltefosine tested negative in the AMES-Salmonella test, DNA-amplification test, chromosomal aberration test in vitro, UDS-test in vivo/in vitro, and oral mouse micronucleus test in vivo. The V 79 mammalian cell HPRT gene mutation test showed an increase in mutant frequency without dose dependency. In view of all mutagenicity test results, the single positive finding in the V 79 HPRT test is considered to be not of toxicological relevance with respect to a mutagenic risk to humans.
Carcinogenicity studies were not performed. In a 52-week oral rat toxicity study, testicular Leydig cell adenoma was observed in 3 of 30 male rats with daily administration of 21.5 mg/kg/day miltefosine (1.0 times the MRHD based on BSA comparison). The carcinogenic potential of miltefosine in humans is unknown.
In a Segment I fertility study in male rats, testicular atrophy, reduced numbers of viable sperm, and impaired fertility were observed in rats following daily oral doses of ≥ 8.25 mg/kg (0.4 times the MRHD based on BSA comparison). These findings were reversible within a recovery period of 10 weeks except at the highest dose tested, 21.5 mg/kg/day (1.0 times the MRHD based on BSA comparison), where effects were not fully reversible.
In a female fertility study in rats, estrus cycle arrest in the metestrus or diestrus phases occurred with the high-dose of 21.5 mg/kg (1.0 times the MRHD based on BSA comparison). At doses of 6.81 and 21.5 mg/kg (0.3 and 1.0 times the MRHD respectively based on BSA comparison) increased numbers of embryonic and fetal resorptions and dead fetuses were observed. In a 52-week toxicology study in dogs, increased numbers of atretic follicles in the ovaries, and cycle arrest in the uterus, vagina, and mammary gland with morphology consistent with anestrus or diestrus was observed at doses ≥ 1 mg/kg/day (0.2 times the MRHD based on BSA comparison). The effects in dogs were fully reversible after a recovery period of 6 weeks.
13.2 Animal Toxicology and/or Pharmacology
Toxicological studies with miltefosine have been performed in mice, rats, dogs, and rabbits. Adverse reactions not observed in clinical studies but seen in animals at exposure levels similar to clinical exposure levels and with possible relevance to clinical use were as follows:
Acute and chronic toxicity: The oral administration of miltefosine in rats was associated with lesions affecting the eyes (retinal degeneration). Retinal degeneration was observed after 8-weeks treatment at doses of 10 mg/kg/day (0.5 times the MRHD based on BSA comparison). Juvenile rats were more sensitive to the miltefosine-induced effects, especially on eyes and kidneys, than adult rats with retinal degeneration occurring at doses ≥ 2.15 mg/kg/day (0.1 times the MRHD based on BSA comparison), and reversible damage to proximal tubule epithelium occurring at doses ≥ 4.64 mg/kg/day (0.2 times the MRHD based on BSA comparison).
14 CLINICAL STUDIES
14.1 Treatment of Visceral Leishmaniasis
One randomized, open-label, active-controlled study was conducted to evaluate the efficacy of IMPAVIDO in the treatment of visceral leishmaniasis in Bihar, India, an area where L. donovani is known epidemiologically to be the prevalent infecting species. Patients ≥ 12 years of age with clinical signs and symptoms compatible with visceral leishmaniasis (fever, splenomegaly, and cytopenia) confirmed by the presence of Leishmania amastigotes in aspirates of spleen or bone marrow were randomized to receive oral IMPAVIDO or intravenous amphotericin B deoxycholate in a 3:1 ratio. Patients weighing ≥ 25 kg received an IMPAVIDO 50 mg capsule with meals twice a day. Patients weighing < 25 kg received an IMPAVIDO 50 mg capsule with meals once a day in the morning. Weight ranged between 15 and 67 kg (mean weight 38.6 kg) and BMI ranged between 8.2 and 24 (mean 16.1). No patient weighed more than 70kg. Amphotericin B was administered intravenously over 6 continuous hours at 1 mg/kg every other day for 15 doses. Patients were hospitalized for the duration of therapy.
Exclusion criteria included platelet count <50 × 109/L, white cell count <1 × 109/L, hemoglobin <6 g/100 mL, AST or ALT ≥3 times upper limit of the normal range, bilirubin ≥2 times upper limit of the normal range, serum creatinine or BUN >1.5 times upper limit of the normal range, prothrombin time >5 seconds above control, and any non-compensated or uncontrolled condition including human immunodeficiency virus (HIV) infection. Women of reproductive potential were required to use effective contraception for the duration of therapy and for 2 months post therapy.
Final cure was defined as initial cure at end of therapy plus absence of signs and symptoms of visceral leishmaniasis at 6 months follow up. Initial cure at the end of therapy was evaluated by repeat spleen or bone marrow aspiration. Patients with initial parasitologic cure were followed for 6 months; patients without absence of clinical signs and symptoms of visceral leishmaniasis were to be evaluated with repeat spleen or bone marrow aspiration to determine final cure.
Two hundred and ninety nine (299) patients received IMPAVIDO and 99 patients received amphotericin B. Approximately, 70% of patients in each arm had previously failed treatment with pentavalent antimony. Initial cure was achieved in 98% of patients in each treatment arm. At 6 months after therapy, 88 (29.5%) IMPAVIDO recipients and 12 (12.1%) amphotericin B recipients continued to have signs and symptoms suggestive of visceral leishmaniasis. These signs or symptoms were attributed to alternative diagnosis in 73 patients. The remaining 27 patients, all in the IMPAVIDO arm, underwent evaluation with splenic or bone marrow aspiration, and 9 (3.0%) were positive for Leishmania amastigotes, indicating relapse. The final cure rates for IMPAVIDO and amphotericin B were 94% and 97%, respectively.
|
||
|
IMPAVIDO |
Amphotericin B Deoxycholate |
|
|
End of therapy |
||
|
Initial Cure |
293 (98%) |
97 (98%) |
|
6 months after therapy |
||
|
Final Cure* |
282 (94%) |
96 (97%) |
|
Treatment Failure |
9 (3%) |
0 (0) |
|
Not Assessable |
8 (3%) |
3 (3%) |
14.2 Treatment of Cutaneous Leishmaniasis
A placebo controlled study was performed in Colombia where L. panamensis and L. braziliensis are epidemiologically known to be the prevalent infecting Leishmania species, and in Guatemala where L. braziliensis is epidemiologically known to be the prevalent infecting species. The study included male and female patients older than 12 years of age who had newly diagnosed or relapsing cutaneous leishmaniasis without mucosal involvement, parasitologically confirmed, presenting with at least one skin ulcer with minimum area of 50 mm2. Exclusion criteria were AST or ALT ≥2 times upper limit of normal range, bilirubin ≥1.5 times upper limit of normal range, and serum creatinine or BUN >1.5 times upper limit of normal range. Women of reproductive potential were required to use effective contraception for the duration of therapy and for 2 months post therapy.
Patients were randomized to receive IMPAVIDO or placebo in a 2:1 allocation. Patients who weighed < 45 kg received IMPAVIDO 50 mg capsule twice a day, and patients who weighed ≥45 kg received IMPAVIDO 50 mg capsule three times a day. No patient weighed more than 84 kg. Definite cure was defined as apparent (complete epithelialization of all lesions) or partial cure (incomplete epithelialization, no enlargement by > 50% in lesions, no appearance of new lesions, and negative parasitology if done) at 2 weeks after end of therapy and complete epithelialization of all ulcers at 6 months after end of therapy. The definite cure rate for IMPAVIDO was statistically significantly higher than the cure rate for placebo.
|
||
|
IMPAVIDO |
Placebo |
|
|
Definite Cure* |
59/89 (66%) |
13/44 (30%) |
|
Colombia |
40/49 (82%) |
9/24 (38%) |
|
Guatemala |
19/40 (48%) |
4/20 (20%) |
An additional study of IMPAVIDO was conducted in Bahia and Manaus, two regions in Brazil where respectively L. braziliensis and L. guyanensis are epidemiologically the prevalent infecting pathogens. Adolescent/adult patients aged 12-65 years received IMPAVIDO orally for 28 days. IMPAVIDO target dose was 2.5 mg/kg/day: patients weighing 15-29 kg received 50 mg once daily, patients weighing 30-45 kg received 50 twice mg daily and patients weighing > 46 kg received 50 mg three times daily. The efficacy criteria were initial cure (complete re-epithelialization of the ulcer at 2 months after the end of therapy) followed by definite cure (complete re-epithelialization at 6 months after the end of therapy). Definite cure rate in patients aged ≥12 years was 27/40 (67.5%) for Manaus, Brazil and 34/40 (85%) for Bahia, Brazil.
14.3 Treatment of Mucosal Leishmaniasis
A single arm study was conducted to evaluate the efficacy of IMPAVIDO capsules for the treatment of mucosal leishmaniasis. The study was conducted in Bolivia where L. braziliensis is epidemiologically the prevalent species.
Seventy nine (79) patients ≥18 years of age with a cutaneous leishmaniasis scar plus parasites observed or cultured from lesion material or a positive skin test, and no clinically significant concomitant disease received miltefosine at a target dose of 2.5 mg/kg/day for 28 days. By 12 months after the end of therapy, 49 of the patients (62%) had complete resolution of edema, erythema, infiltration and erosion from the involved mucosal sites.
16 HOW SUPPLIED/STORAGE AND HANDLING
Each IMPAVIDO capsule contains 50 mg miltefosine in an opaque, red, hard gelatin capsule. IMPAVIDO capsules are supplied in a folded peel/push-through blister card. Each blister card contains 14 capsules. Each carton contains two blister cards (NDC 61744-050-01).
Store at 20-25 °C (68-77 °F); excursions permitted to 15-30 °C (59-86 °F). [See USP Controlled Room Temperature]. Protect from moisture.
Dispense only in the original carton.
17 PATIENT COUNSELING INFORMATION
See the FDA-approved Medication Guide
17.1 Dosing Instructions
- •
- IMPAVIDO is administered with food to ameliorate gastrointestinal side effects.
- •
- Instruct the patient to swallow the capsule whole and not to chew it or break it apart. Instruct the patient to complete the full course of therapy.
- •
- Inform the patient that abdominal pain, nausea, vomiting, and diarrhea are common side effects of therapy with IMPAVIDO and instruct the patient to inform their healthcare provider if these gastrointestinal side effects are severe or persistent. Instruct the patient to consume sufficient fluids to avoid dehydration and, consequently, the risk of kidney injury.
17.2 Females and Males of Reproductive Potential
- •
- Advise women of reproductive potential to use effective contraception during IMPAVIDO therapy and for 5 months after therapy ends [see Boxed Warning and Use in Specific Populations (8.1, 8.8)].
- •
- Advise women who use oral contraceptives to use additional non-hormonal or alternative method(s) of effective contraception during IMPAVIDO therapy if vomiting and/or diarrhea occurs [see Warnings and Precautions (5.7) and Use in Specific Populations (8.8)].
- •
- Advise nursing mothers not to breastfeed during IMPAVIDO therapy and for 5 months after therapy is completed [see Use in Specific Populations (8.3)].
- •
- Advise women and men that IMPAVIDO caused infertility in male rats, impaired fertility in female rats, and caused atresia in ovarian follicles in female dogs. Advise patients that the potential of impaired fertility in humans has not been adequately evaluated [see Warnings and Precautions (5.2) and Use in Specific Populations (8.8)].
Distributed by:
Paladin Therapeutics Inc.
Corporation Trust Center
1209 Orange Street
Wilmington, DE 19801
USA
Medication Guide
IMPAVIDO® (Im-PA-vee-do)
(miltefosine)
Capsules
Read this Medication Guide before you receive IMPAVIDO. This information does not take the place of talking to your healthcare provider about you medical condition or treatment.
What is the most important information I should know about IMPAVIDO?
IMPAVIDO may cause serious risks to pregnancy:
- •
- Do not take IMPAVIDO if you are pregnant. If you take IMPAVIDO during pregnancy, your baby is at risk for death or serious birth defects.
- •
- Women who can become pregnant should use effective birth control (contraception) during IMPAVIDO treatment and for 5 months after stopping IMPAVIDO treatment. Discuss with your healthcare provider which birth control method is right for you.
- •
- If you become pregnant while taking IMPAVIDO, tell your healthcare provider right away. Talk to your healthcare provider about taking part in the IMPAVIDO Pregnancy Registry. This is a study to learn how IMPAVIDO affects pregnancy and babies. You can enroll in this registry by calling xxxxxxx.
What is IMPAVIDO?
IMPAVIDO is prescription medicine used to treat certain types of leishmaniasis:
- •
- visceral leishmaniasis (affecting your internal organs)
- •
- cutaneous leishmaniasis (affecting the skin)
- •
- mucosal leishmaniasis (affecting the nose, mouth and throat)
It is not known if IMPAVIDO is safe and effective in children under 12 years of age.
Who should not take IMPAVIDO? Do not take IMPAVIDO if you:
- •
- are pregnant
- •
- have Sjögren-Larsson-Syndrome
- •
- are allergic to miltefosine or any of the ingredients in IMPAVIDO. See the end of this leaflet for a list of the ingredients in IMPAVIDO.
- •
- are a woman who can become pregnant and have not had a pregnancy test. Women who can get pregnant must have a urine or blood pregnancy test before taking IMPAVIDO.
- •
- are a woman who can become pregnant and you are not willing to use effective birth control during IMPAVIDO treatment and for 5 months after treatment
Before you take IMPAVIDO, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have kidney or liver problems. Your healthcare provider should do blood tests to check your kidneys and liver before you start, during and after your treatment with IMPAVIDO.
- •
- are pregnant or planning to become pregnant. IMPAVIDO may harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. It is not known if IMPAVIDO passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take IMPAVIDO. You should avoid breastfeeding while you take IMPAVIDO and for 5 months after you stop taking IMPAVIDO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take IMPAVIDO?
- •
- Take IMPAVIDO exactly as your healthcare provider tells you to.
- •
- Complete your full 28 day IMPAVIDO treatment.
- •
- Take IMPAVIDO capsules whole. Do not break, crush, dissolve, or chew IMPAVIDO before swallowing.
- •
- Take IMPAVIDO with food to help reduce stomach problems.
What are the possible side effects of IMPAVIDO?
IMPAVIDO may cause serious side effects, including:
See “What is the most important information I should know about IMPAVIDO?”
- •
- Fertility problems in male and female rats and abnormal menstrual cycle in female dogs. It is not known if IMPAVIDO causes fertility problems in men or women
- •
- Testicular pain and absent or decreased ejaculation
- •
- Kidney and liver problems
- •
- Stomach problems. IMPAVIDO can cause vomiting, diarrhea, and dehydration. Call your healthcare provider right away if you have severe vomiting and diarrhea that does not go away. Drink a lot of water to help prevent dehydration while you are having vomiting and diarrhea.
- •
- Decreased effectiveness of oral contraceptive pills. Vomiting and diarrhea may cause your birth control pills to be less effective at preventing pregnancy. Use an extra method of birth control, such as male condoms with spermicide, until you are no longer having vomiting and diarrhea.
- •
- Decrease in platelets (which are blood cells that help blood clot).
- •
- Serious Skin Problems. IMPAVIDO can cause a serious skin problem called Stevens-Johnson Syndrome. If you develop a skin rash with blisters while taking IMPAVIDO, stop taking IMPAVIDO right away and call your healthcare provider.
The most common side effects of IMPAVIDO include: nausea, vomiting and diarrhea. Other side effects include abdominal pain, decreased appetite, dizziness, headache, sleepiness, skin itching, and abnormalities in liver or kidney tests.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of IMPAVIDO. For more information, ask your healthcare provider.
You may report side effects to FDA at 1-800-FDA-1088.
How should I store IMPAVIDO?
- •
- Store IMPAVIDO at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Protect IMPAVIDO from moisture.
Keep IMPAVIDO and all medicines out of the reach of children.
General information about the safe and effective use of IMPAVIDO.
Medicines are sometimes prescribed for purposes other than those listed in this Medication Guide. Do not use IMPAVIDO for a condition for which it was not prescribed. This Medication Guide summarizes the most important information about IMPAVIDO. If you would like more information, talk to your healthcare provider or pharmacist. You can ask your healthcare or pharmacist for information about IMPAVIDO that is written for health professionals. Do not give IMPAVIDO to other people, even if they have the same symptoms that you have. For more information, go to www.TRADENAME.com or www.dailymed.nlm.nih.gov, or call 1-XXX-XXX-XXXX.
What are the ingredients in IMPAVIDO?
Active ingredient: miltefosine
Inactive ingredients: colloidal silicon dioxide, microcrystalline cellulose, lactose monohydrate, talc, and magnesium stearate. The capsule shell contains gelatin, titanium dioxide, ferric oxide, and purified water.
Distributed by: Paladin Therapeutics, Inc.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Issued: March 2014
PRINCIPAL DISPLAY PANEL - CARTON
NDC 61744-050-01 Paladin Therapeutics
Impavido®
(miltefosine) capsules
50 mg per Capsule
Rx only 28 capsules
Contains 2 blister cards, 14 blisters per card, 1 capsule per blister.
Active ingredient: miltefosine
Inactive ingredients: colloidal silicon dioxide, lactose monohydrate,
magnesium stearate, microcrystalline cellulose, talc. The capsule
shell contains gelatin, titanium dioxide ferric oxide and purified water.
Usual dosage: see enclosed package insert for complete
prescribing information.
Keep out of reach of chidren.
Store at 20-25 °C (68-77 °F); excursions permitted
to 15-30 °C (59-86 °F). [See USP Controlled Room Temperature].
Distributed by:
Paladin Therapeutics Inc.
Corporation Trust Center
1209 Orange Street
Wilmington, DE
USA 19801
| IMPAVIDO
miltefosine capsule |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Paladin Therapeutics Inc. (832989383) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Siegfried Evionnaz SA | 480093164 | API MANUFACTURE(61744-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Haupt Pharma Amareg GmbH | 331334909 | MANUFACTURE(61744-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Carton Service, Inc. | 928861723 | PACK(61744-050) | |