LIDOCAINE HCL 4 PERCENT AND MENTHOL 4 PERCENT- lidocaine hcl and menthol spray
7T Pharma LLC
----------
Lidocaine and Menthol Topical Spray
LIDOCAINE AND MENTHOL - Lidocaine HCl 4% and Menthol 4% topical spray
7T Pharma, LLC
----------
Lidocaine 4% and Menthol 4% Topical Spray
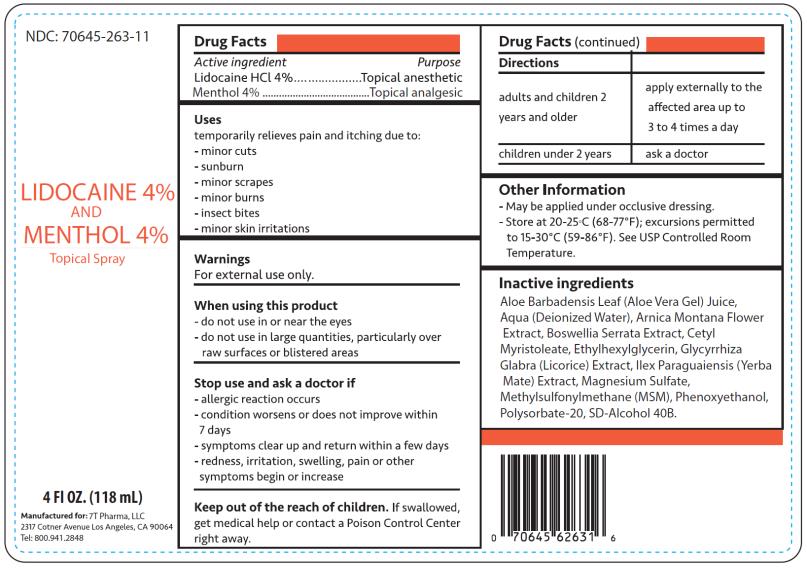
Drug Facts
Uses
Temporarily relieves pain and itching due to:
- minor cuts
- sunburn
- minor scrapes
- minor burns
- insect bites
- minor skin irritations
Warnings
For external use only.
When using this product
- do not use in or near the eyes
- do not use in large quantities, particularly over raw surfaces or blistered areas
Directions
| adults and children 2 years and older | apply externally to the affected area up to 3 to 4 times a day |
| children under 2 years | ask a doctor |
Other information
- May be applied under occlusive dressing.
- Store at 20-25°C (68-77°F); excursions permitted to 15-30°C (59-86°F). See USP Controlled Room Temperature.
Inactive ingredients
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Arnica Montana Flower Extract, Boswellia Serrata Extract, Cetyl Myristoleate, Ethylhexylglycerin, Glycyrrhiza Glabra (Licorice) Extract, Ilex Paraguaiensis (Yerba Mate) Extract, Magnesium Sulfate, Methylsulfonylmethane (MSM), Phenoxyethanol, Polysorbate-20, SD-Alcohol 40B
Lidocaine 4% and Menthol 4% Topical Spray
NDC 70645-263-11
4 fl oz (118mL)
Manufactured for:
7T Pharma, LLC
Los Angeles, CA 90064
| LIDOCAINE HCL 4 PERCENT AND MENTHOL 4 PERCENT
lidocaine hcl and menthol spray |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - 7T Pharma LLC (080220022) |