B-NEXA- pyridoxine, folic acid, calcium, and ginger tablet
Upsher-Smith Laboratories, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
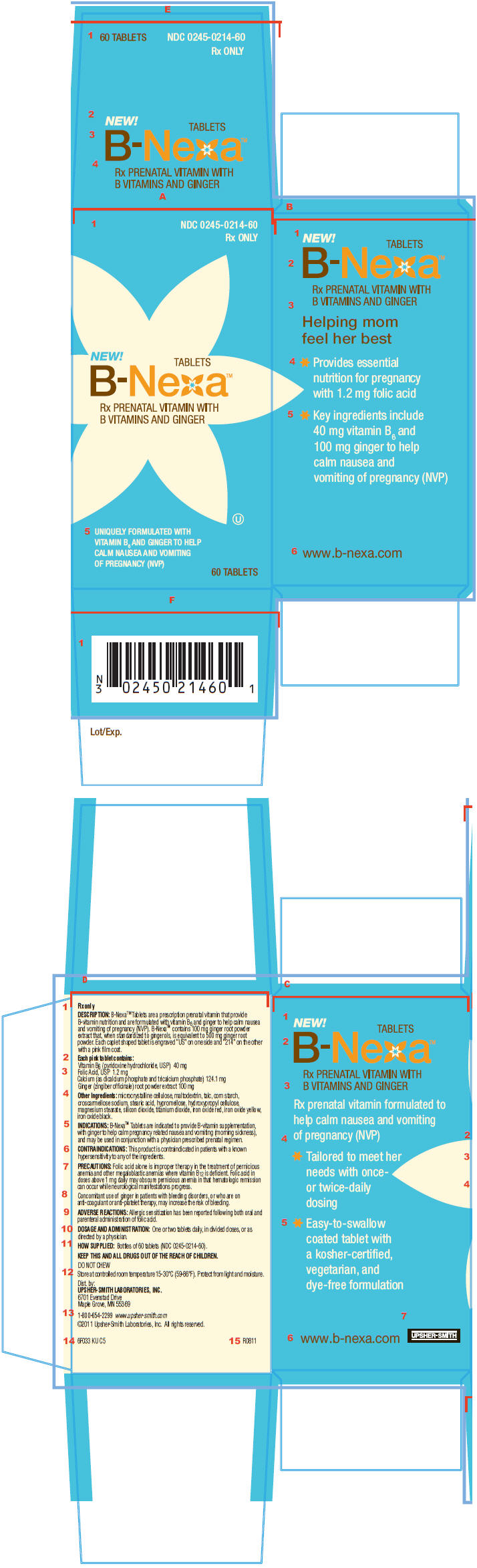
B-NEXA® TABLETS
DESCRIPTION
B-Nexa® Tablets are a prescription prenatal vitamin that provide B-vitamin nutrition and are formulated with vitamin B6 and ginger to help calm nausea and vomiting of pregnancy (NVP). B-Nexa® Tablets contain 100 mg ginger root powder extract that, when standardized to gingerols, is equivalent to 500 mg ginger root powder. Each caplet shaped tablet is engraved "US" on one side and "214" on the other with a pink film coat.
Each pink tablet contains:
| Vitamin B6 (pyridoxine hydrochloride, USP) | 40 mg |
| Folic Acid, USP | 1.2 mg |
| Calcium (as dicalcium phosphate and tricalcium phosphate) | 124.1 mg |
| Ginger (zingiber officinale) root powder extract | 100 mg |
Other Ingredients: microcrystalline cellulose, maltodextrin, talc, corn starch, croscarmellose sodium, stearic acid, hypromellose, hydroxypropyl cellulose, magnesium stearate, silicon dioxide, titanium dioxide, iron oxide red, iron oxide yellow, iron oxide black.
INDICATIONS
B-Nexa® Tablets are indicated to provide B-vitamin supplementation, with ginger to help calm pregnancy related nausea and vomiting (morning sickness), and may be used in conjunction with a physician prescribed prenatal regimen.
CONTRAINDICATIONS
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
PRECAUTIONS
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
Concomitant use of ginger in patients with bleeding disorders, or who are on anti-coagulant or anti-platelet therapy, may increase the risk of bleeding.
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
DOSAGE AND ADMINISTRATION
One or two tablets daily, in divided doses, or as directed by a physician.
| B-NEXA
pyridoxine, folic acid, calcium, and ginger tablet |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Upsher-Smith Laboratories, Inc. (047251004) |