Ask a doctor before use if injuries are
• deep wounds
• puncture wounds
• serious burns
Stop use and ask a doctor if infection occurs or if redness,irritation, swelling or pain persists or increases.
Directions
Apply locally as needed.
Do not use
• if allergic to iodine
• in the eyes
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or consult a poison control center immediately.
For external use only
Avoid excessive heat. Store at room temperature.
Other information
• titratable iodine
• latex free
• for hospital of professsional use only
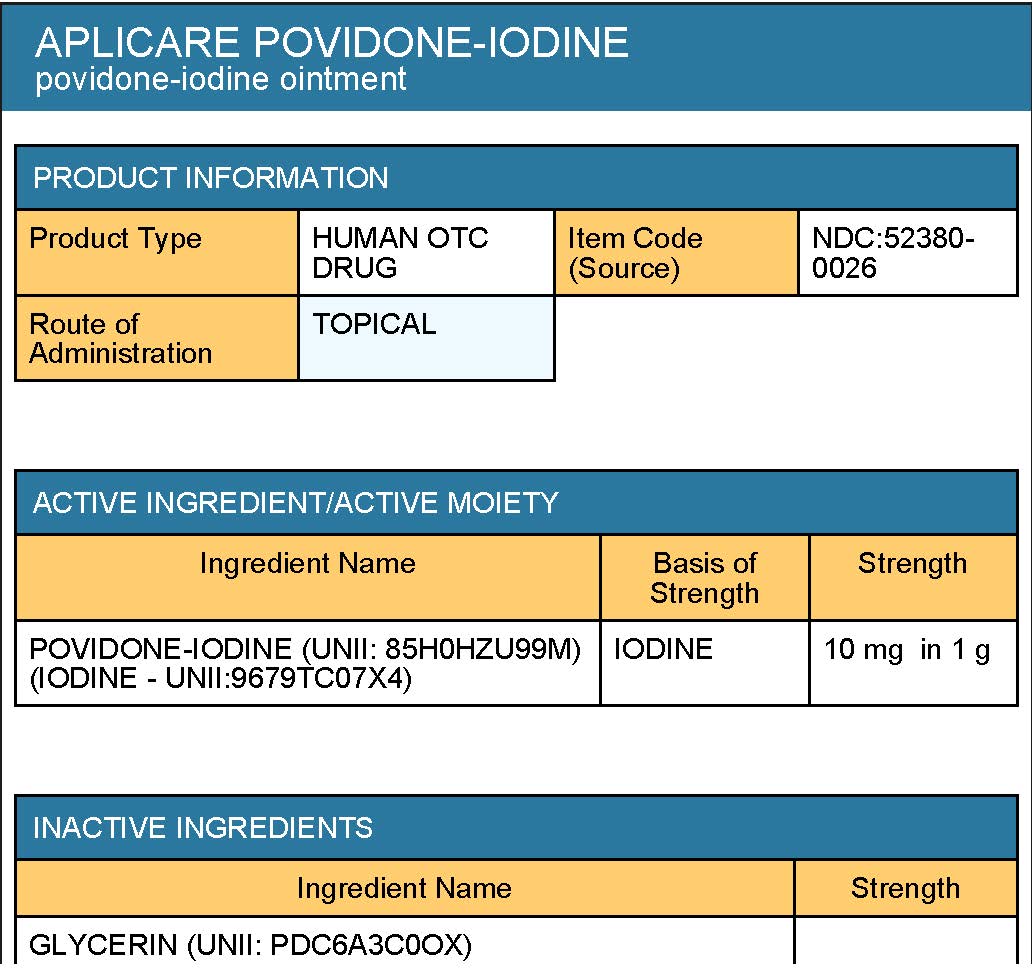
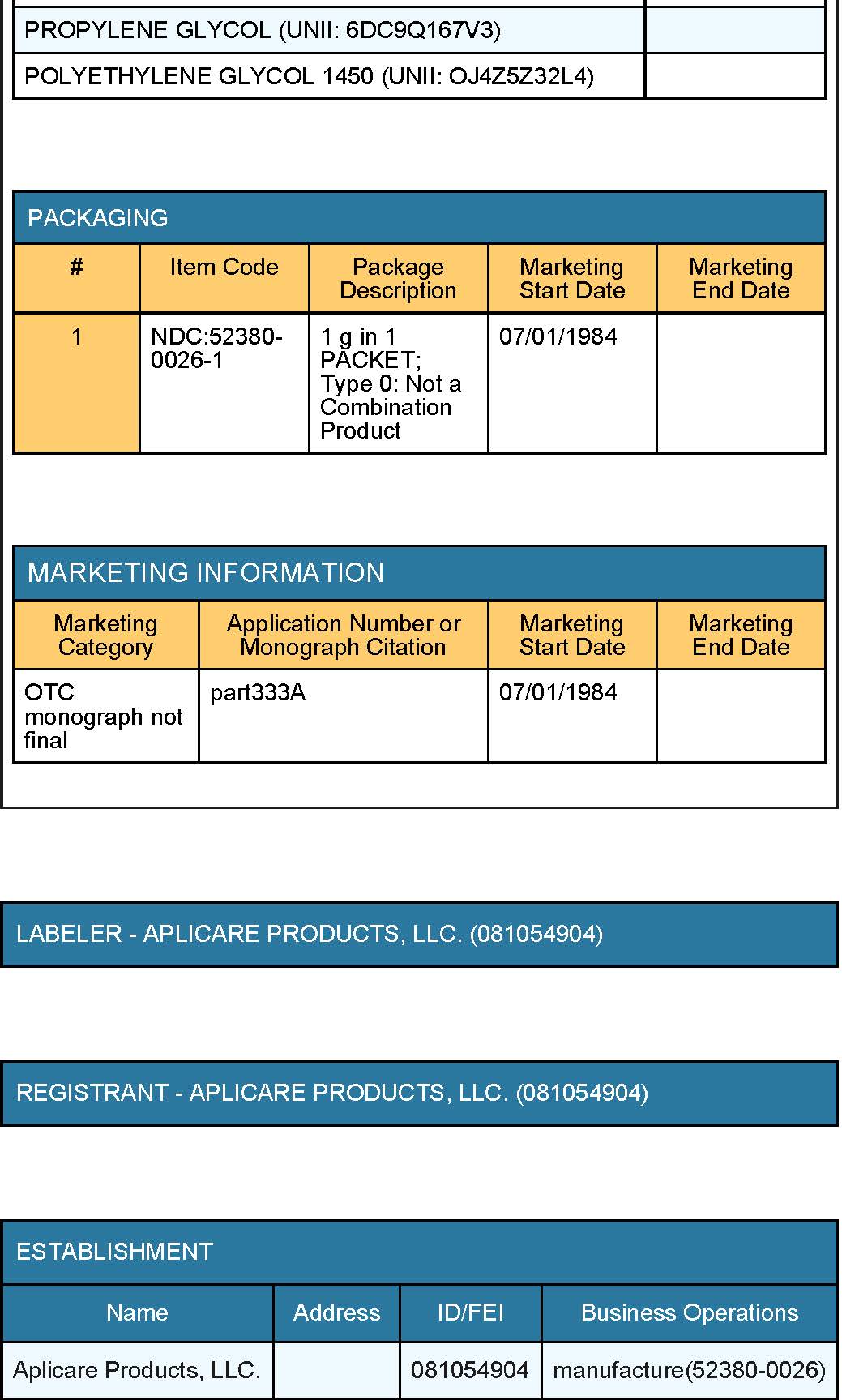
Inactive Ingredients
• polyethylene glycol 1450
• glycerin
• propylene glycol
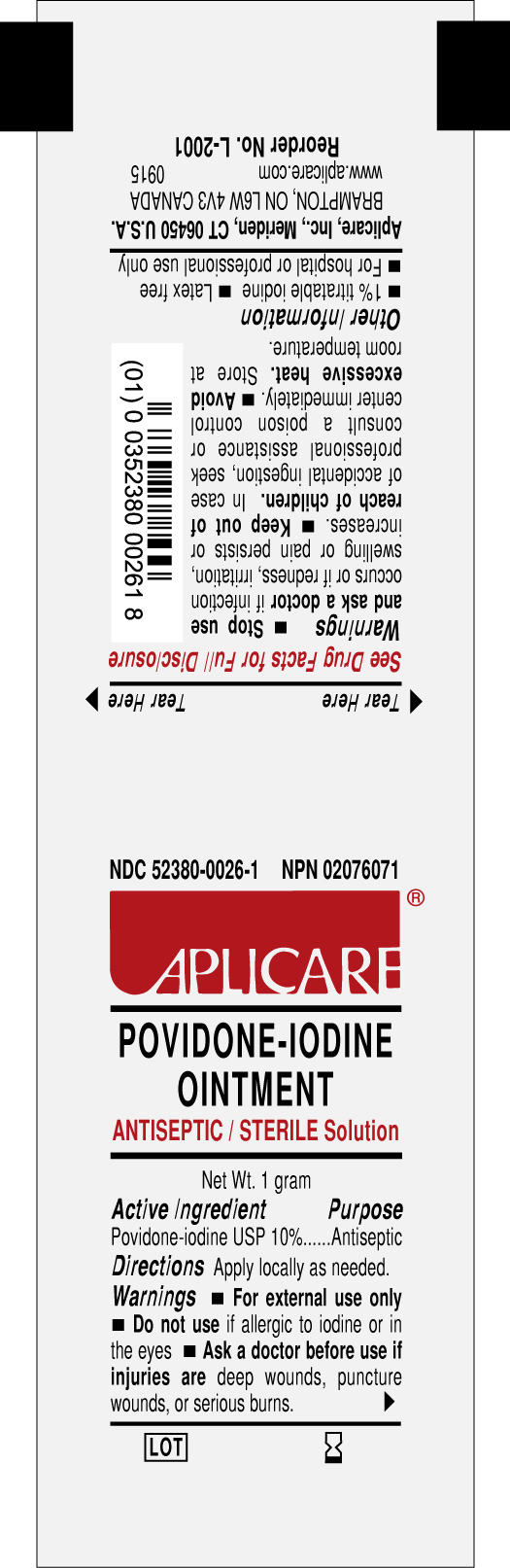
APLICARE
POVIDONE-IODINE OINTMENT
ANTISEPTIC
STERILE SOLUTION
antiseptic skin preparation
Do not use
- if allergic to iodine
- in the eyes
For external use only
Ask a doctor before use if injuries are
- deep or puncture wounds
- serious burns
Stop use and ask a doctor if
- redness, irritation, swelling or pain persists or increases
- infection occurs
Avoid pooling beneath patient
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or consult a poison control center immediately.
Avoid excessive heat. Store at room temperature.
- 1% titratable iodine
- not made with natural rubber latex
- for hospital or professional use only
- for single use only
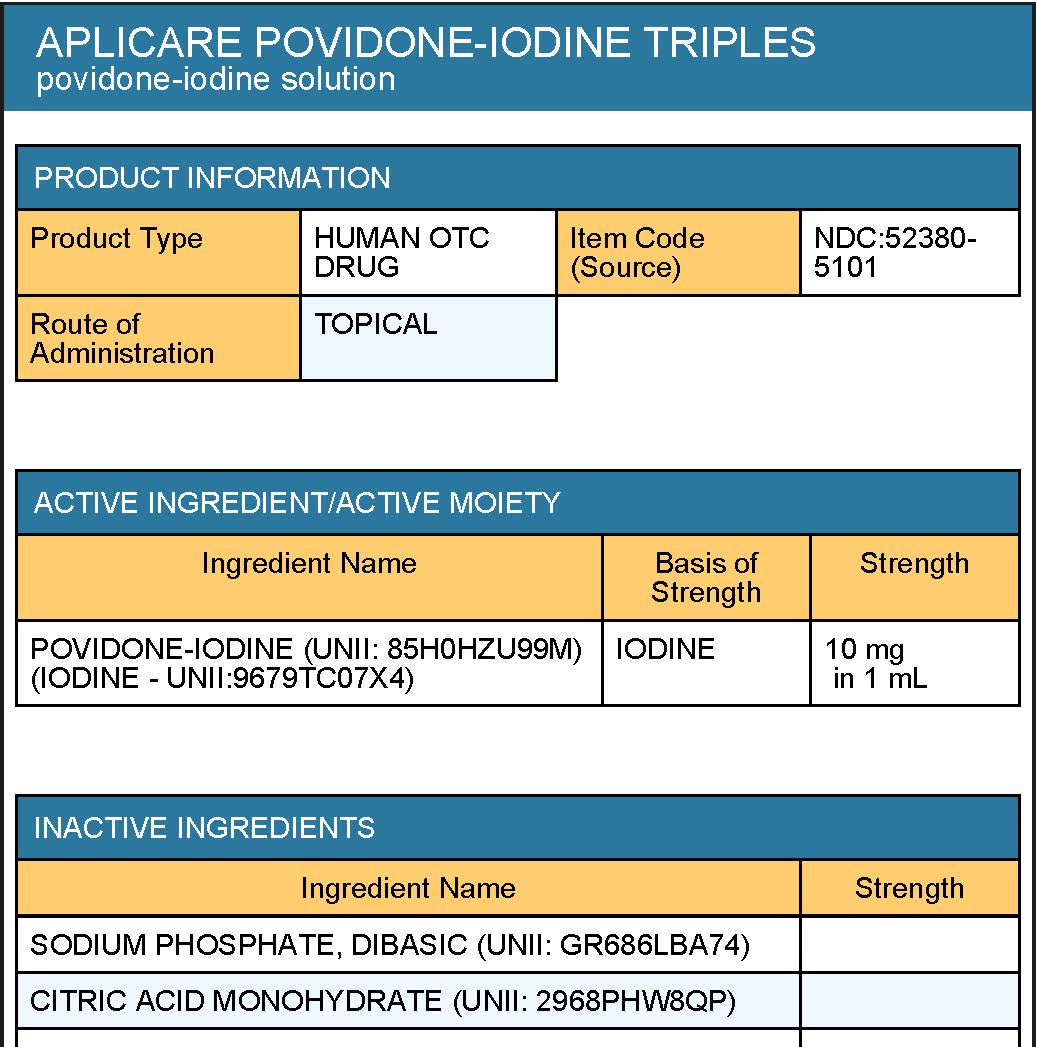
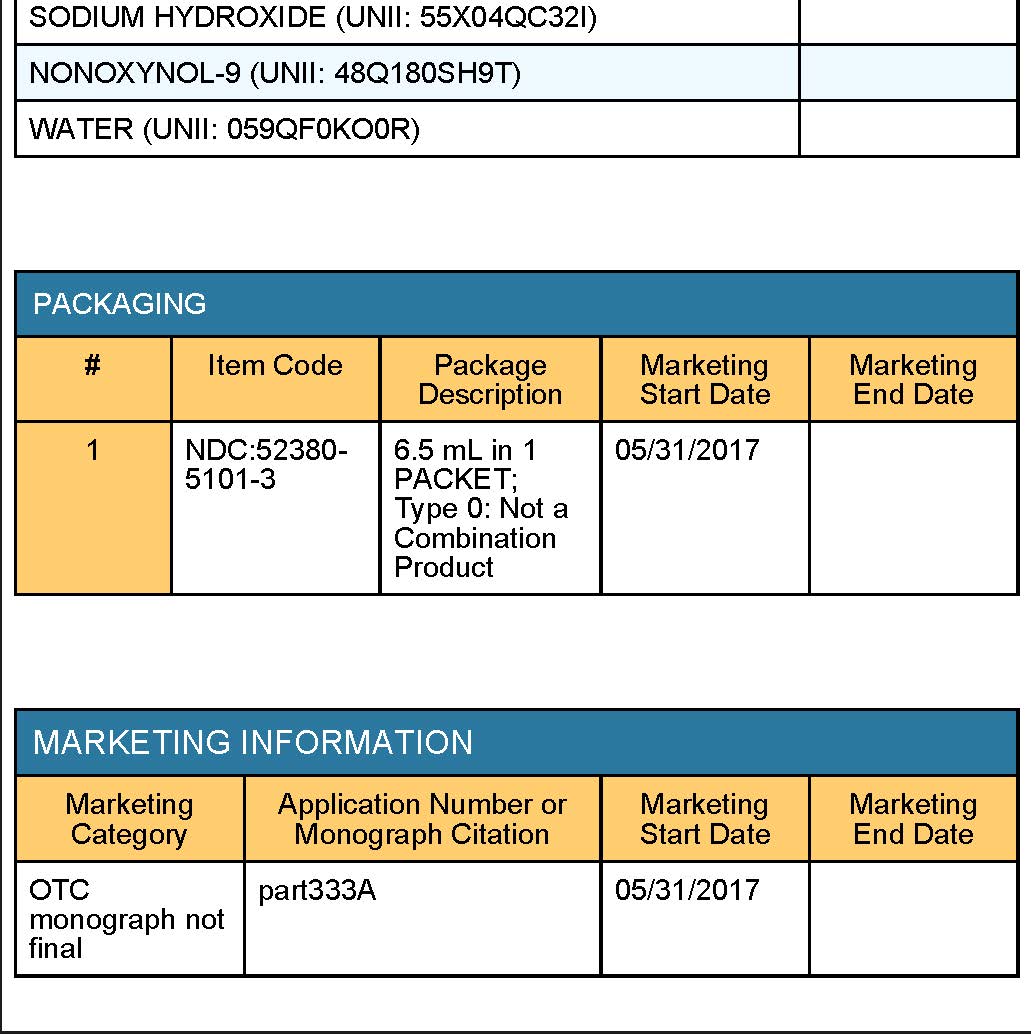
citric acid, disodium phosphate, nonoxynol-9, sodium hydroxide, water
For questions, comments, or to report serious side effects:
800-760-3236
Monday-Friday 8:30 a.m.-5:00 p.m. EST
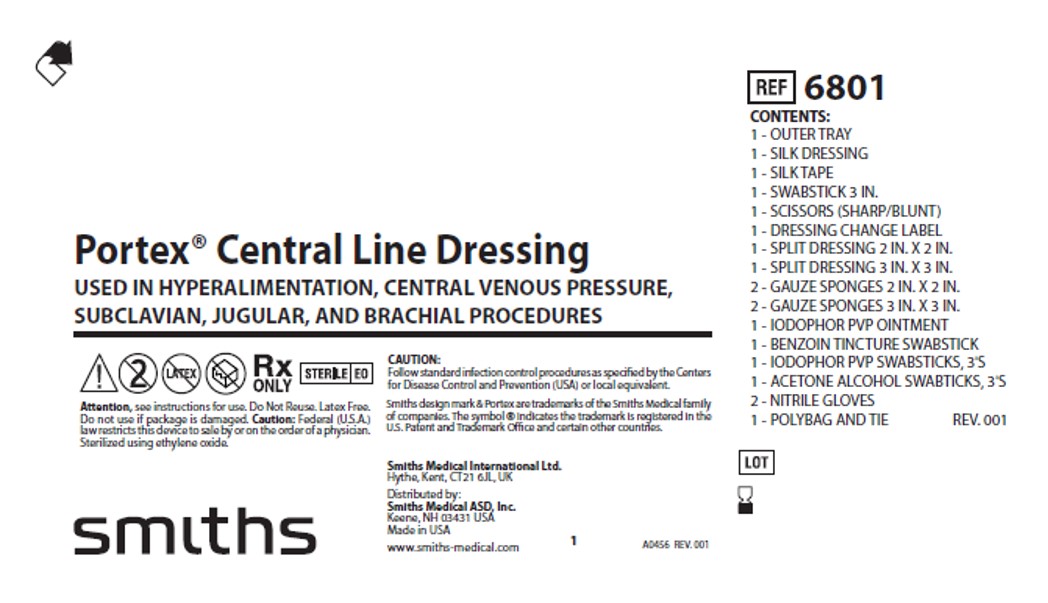
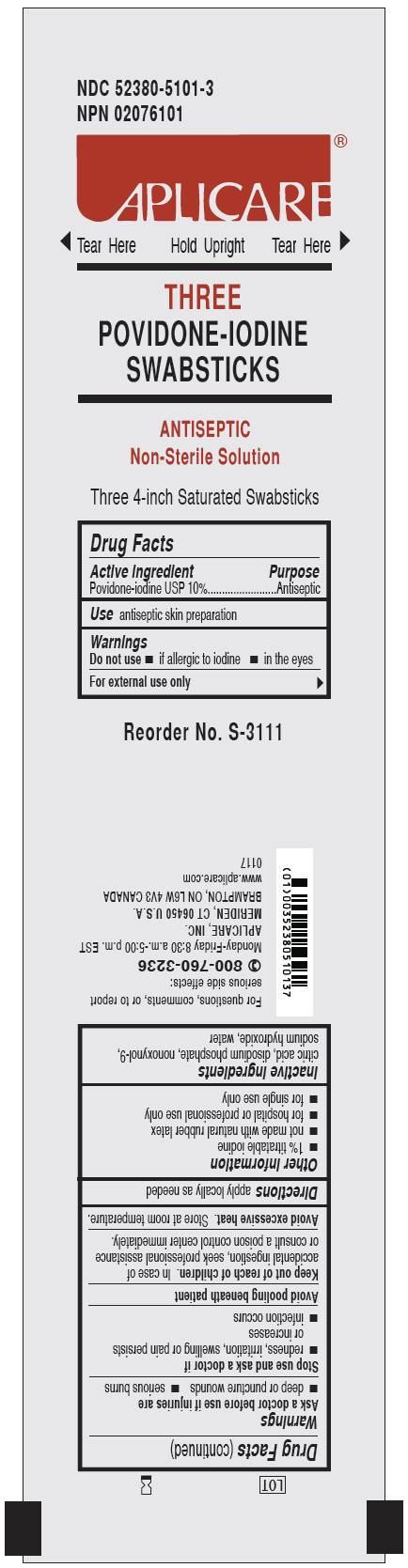
PRINCIPAL DISPLAY PANEL - 3 SWABSTICK PACKET
NDC 52380-5101-3
NPN 02076101
APLICARE
◀ Tear Here Hold Upright Tear Here ▶
THREE
POVIDONE-IODINE
SWABSTICKS
ANTISEPTIC
Non-Sterile Solution
Three 4-inch Saturated Swabsticks
Reorder No. S-3111

Smiths Medical ASD, Inc.