Label: SODIUM CHLORIDE-
SODIUM CHLORIDE-
SODIUM CHLORIDE-
- NHRIC Code(s): 0378-6985-01, 0378-6986-01, 0378-6987-89
- Packager: Mylan Pharmaceuticals Inc.
- Category: MEDICAL DEVICE
Drug Label Information
Updated December 2, 2016
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
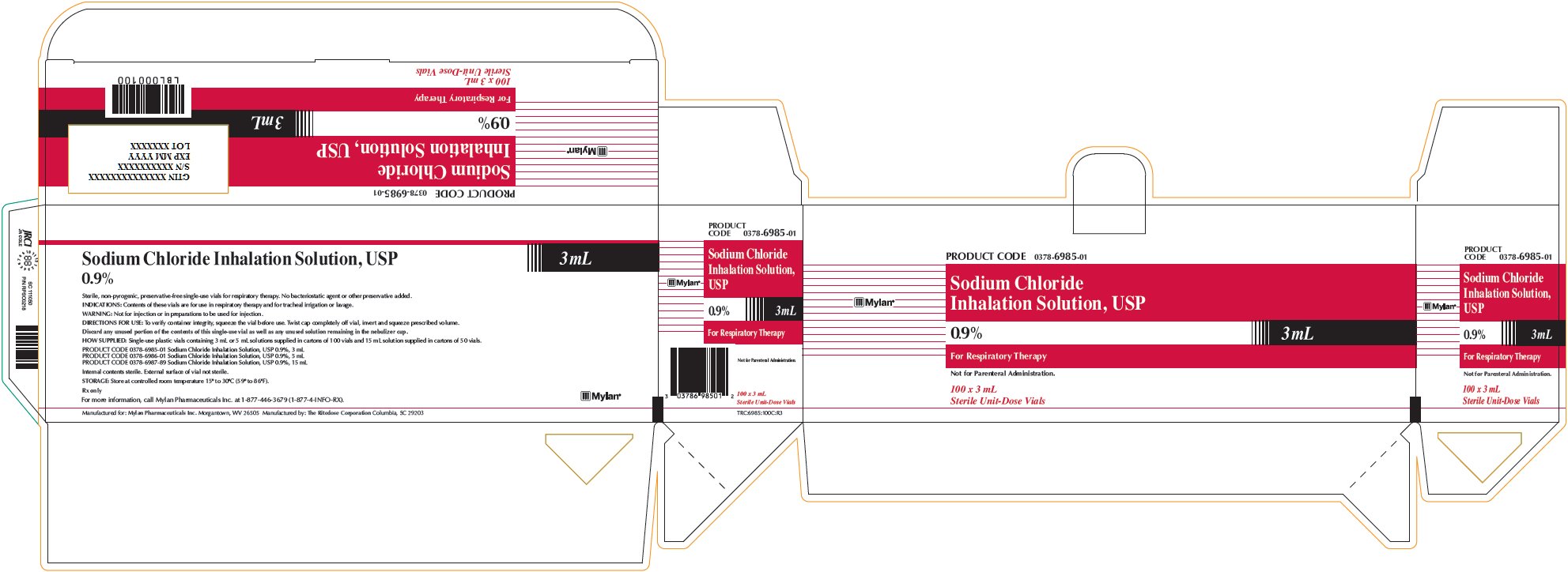
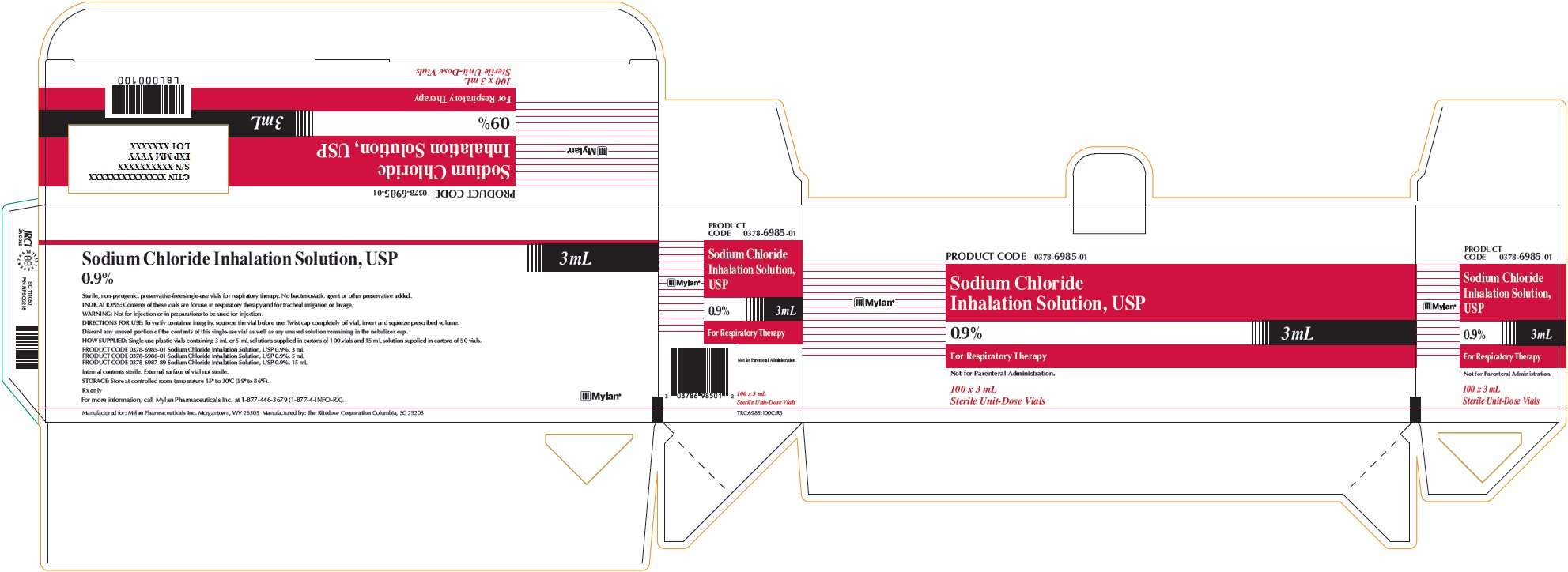
Sodium Chloride Inhalation Solution 0.9% 3 mL
PRODUCT CODE 0378-6985-01
Sodium Chloride
Inhalation Solution, USP
0.9% 3 mLFor Respiratory Therapy
Not for Parenteral Administration.
100 x 3 mL
Sterile Unit-Dose VialsSodium Chloride Inhalation Solution, USP
0.9% 3 mLSterile, non-pyrogenic, preservative-free single-use vials for respiratory therapy. No bacteriostatic agent or other preservative added.
INDICATIONS: Contents of these vials are for use in respiratory therapy and for tracheal irrigation or lavage.
WARNING: Not for injection or in preparations to be used for injection.
DIRECTIONS FOR USE: To verify container integrity, squeeze the vial before use. Twist cap completely off vial, invert and squeeze prescribed volume.
Discard any unused portion of the contents of this single-use vial as well as any unused solution remaining in the nebulizer cup.
HOW SUPPLIED: Single-use plastic vials containing 3 mL or 5 mL solutions supplied in cartons of 100 vials and 15 mL solution supplied in cartons of 50 vials.
PRODUCT CODE 0378-6985-01 Sodium Chloride Inhalation Solution, USP 0.9%, 3 mL
PRODUCT CODE 0378-6986-01 Sodium Chloride Inhalation Solution, USP 0.9%, 5 mL
PRODUCT CODE 0378-6987-89 Sodium Chloride Inhalation Solution, USP 0.9%, 15 mLInternal contents sterile. External surface of vial not sterile.
STORAGE: Store at controlled room temperature 15° to 30°C (59° to 86°F).
Rx only
For more information, call Mylan Pharmaceuticals Inc. at 1-877-446-3679 (1-877-4-INFO-RX).
Manufactured for: Mylan Pharmaceuticals Inc. Morgantown, WV 26505 Manufactured by: The Ritedose Corporation Columbia, SC 29203
TRC:6985:100C:R3
-
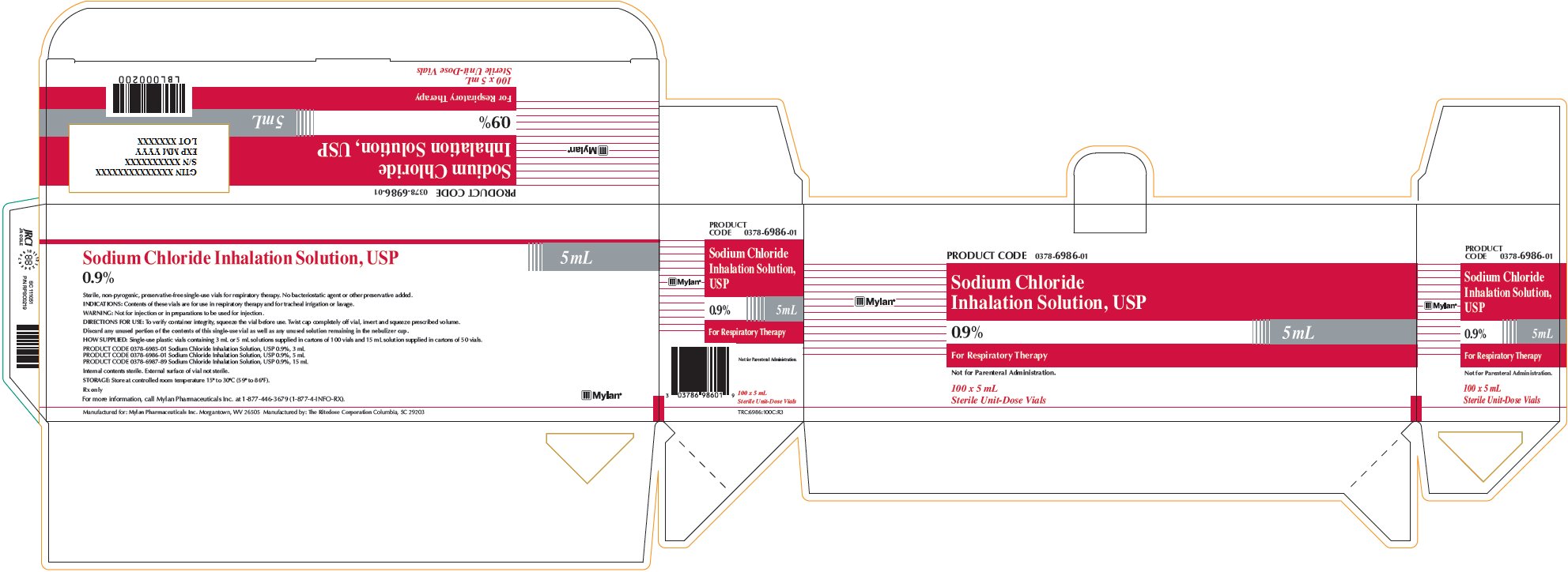
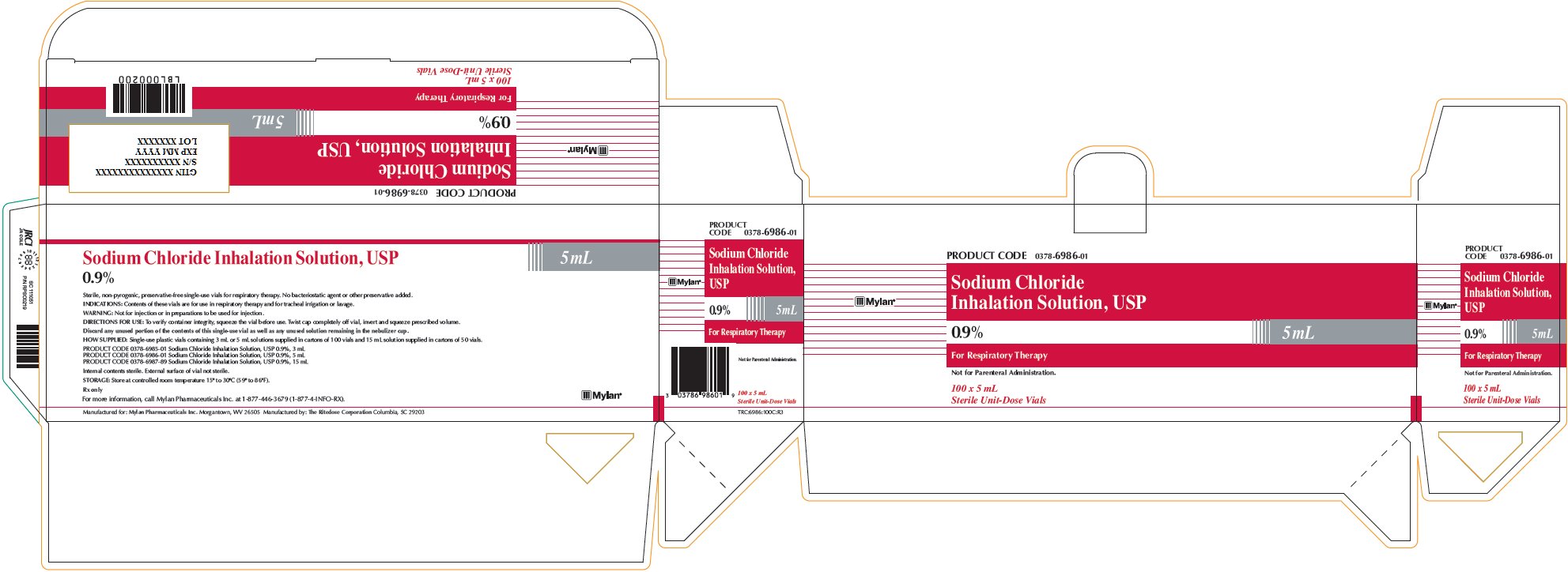
Sodium Chloride Inhalation Solution 0.9% 5 mL
PRODUCT CODE 0378-6986-01
Sodium Chloride
Inhalation Solution, USP
0.9% 5 mLFor Respiratory Therapy
Not for Parenteral Administration.
100 x 5 mL
Sterile Unit-Dose VialsSodium Chloride Inhalation Solution, USP
0.9% 5 mLSterile, non-pyrogenic, preservative-free single-use vials for respiratory therapy. No bacteriostatic agent or other preservative added.
INDICATIONS: Contents of these vials are for use in respiratory therapy and for tracheal irrigation or lavage.
WARNING: Not for injection or in preparations to be used for injection.
DIRECTIONS FOR USE: To verify container integrity, squeeze the vial before use. Twist cap completely off vial, invert and squeeze prescribed volume.
Discard any unused portion of the contents of this single-use vial as well as any unused solution remaining in the nebulizer cup.
HOW SUPPLIED: Single-use plastic vials containing 3 mL or 5 mL solutions supplied in cartons of 100 vials and 15 mL solution supplied in cartons of 50 vials.
PRODUCT CODE 0378-6985-01 Sodium Chloride Inhalation Solution, USP 0.9%, 3 mL
PRODUCT CODE 0378-6986-01 Sodium Chloride Inhalation Solution, USP 0.9%, 5 mL
PRODUCT CODE 0378-6987-89 Sodium Chloride Inhalation Solution, USP 0.9%, 15 mLInternal contents sterile. External surface of vial not sterile.
STORAGE: Store at controlled room temperature 15° to 30°C (59° to 86°F).
Rx only
For more information, call Mylan Pharmaceuticals Inc. at 1-877-446-3679 (1-877-4-INFO-RX).
Manufactured for: Mylan Pharmaceuticals Inc. Morgantown, WV 26505 Manufactured by: The Ritedose Corporation Columbia, SC 29203
TRC:6986:100C:R3
-
Sodium Chloride Inhalation Solution 0.9% 15 mL
PRODUCT CODE 0378-6987-89
Sodium Chloride
Inhalation Solution, USP
0.9% 15 mLFor Respiratory Therapy
Not for parenteral administration.
50 x 15 mL
Sterile Unit-Dose VialsSodium Chloride Inhalation Solution, USP
0.9% 15 mLSterile, non-pyrogenic, preservative-free single-use vials for respiratory therapy. No bacteriostatic agent or other preservative added.
INDICATIONS: Contents of these vials are for use in respiratory therapy and for tracheal irrigation or lavage.
WARNING: Not for injection or in preparations to be used for injection.
DIRECTIONS FOR USE: To verify container integrity, squeeze the vial before use. Twist cap completely off vial, invert and squeeze prescribed volume.
Discard any unused portion of the contents of this single-use vial as well as any unused solution remaining in the nebulizer cup.
HOW SUPPLIED: Single-use plastic vials containing 3 mL or 5 mL solutions supplied in cartons of 100 vials and 15 mL solution supplied in cartons of 50 vials.
PRODUCT CODE 0378-6985-01 Sodium Chloride Inhalation Solution, USP 0.9%, 3 mL
PRODUCT CODE 0378-6986-01 Sodium Chloride Inhalation Solution, USP 0.9%, 5 mL
PRODUCT CODE 0378-6987-89 Sodium Chloride Inhalation Solution, USP 0.9%, 15 mLInternal contents sterile. External surface of vial not sterile.
STORAGE: Store at controlled room temperature 15° to 30°C (59° to 86°F).
Rx only
For more information, call Mylan Pharmaceuticals Inc. at 1-877-446-3679 (1-877-4-INFO-RX).
Manufactured for: Mylan Pharmaceuticals Inc., Morgantown, WV 26505 Manufactured by: The Ritedose Corporation Columbia, SC 29203
TRC:6987:50C:R3
-
INGREDIENTS AND APPEARANCE
SODIUM CHLORIDE
nebulizer (direct patient interface)Product Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:0378-6985 Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 0.027 g in 3 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:0378-6985-01 100 in 1 CARTON 1 3 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Exempt device CCQ 10/12/2009 SODIUM CHLORIDE
nebulizer (direct patient interface)Product Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:0378-6986 Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 0.045 g in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:0378-6986-01 100 in 1 CARTON 1 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Exempt device CCQ 10/12/2009 SODIUM CHLORIDE
nebulizer (direct patient interface)Product Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:0378-6987 Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 0.135 g in 15 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:0378-6987-89 50 in 1 CARTON 1 15 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Exempt device CCQ 10/12/2009 Labeler - Mylan Pharmaceuticals Inc. (059295980)