BLUE GEL PAIN RELIEVING- menthol gel
Rugby Laboratories
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
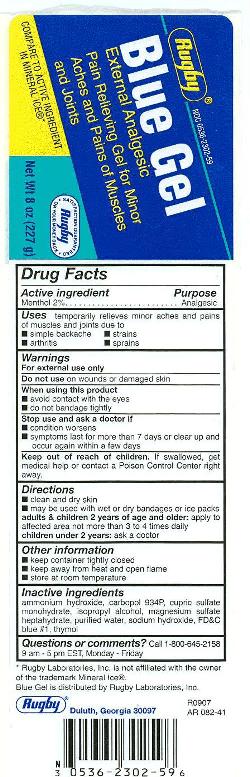
Drug Facts
Uses
● temporarily relieves minor aches and pains of muscles and joints associated with:
● arthritis ● simple backache ● strains ● sprains
● provides cooling penetrating relief
Stop use and ask a doctor if
- •
- condition worsens
- •
- symptoms last more than 7 days or clear up and occur again within a few days
Keep Out of Reach of Children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- •
- clean and dry skin
- •
- may be used with wet or dry dandages or ice packs
- •
- adults & children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- •
- children under 2 years: ask a doctor
Other information
- •
- store at room temperature
- •
- keep container tightly closed
- •
- keep away from heat or open flame
Inactive ingredients
ammonium hydroxide, carbopol 934P, cupric sulfate monohydrate, FD&C blue no. 1, isopropyl alcohol, magnesium sulfate heptyhydrate, sodium hydroxide, thymol, water
| BLUE GEL
PAIN RELIEVING
menthol gel |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Rugby Laboratories (191427277) |
Revised: 9/2019
Document Id: 7b46d414-ca8e-43ab-8cd6-8939548647b4
Set id: d4e432b1-8997-412f-9357-c7b6757852e5
Version: 2
Effective Time: 20190904
Rugby Laboratories