Label: BISMUTH SUBSALICYLATE tablet, film coated
- NDC Code(s): 70000-0594-1
- Packager: Cardinal Health
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 22, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
- Uses
-
WARNINGS
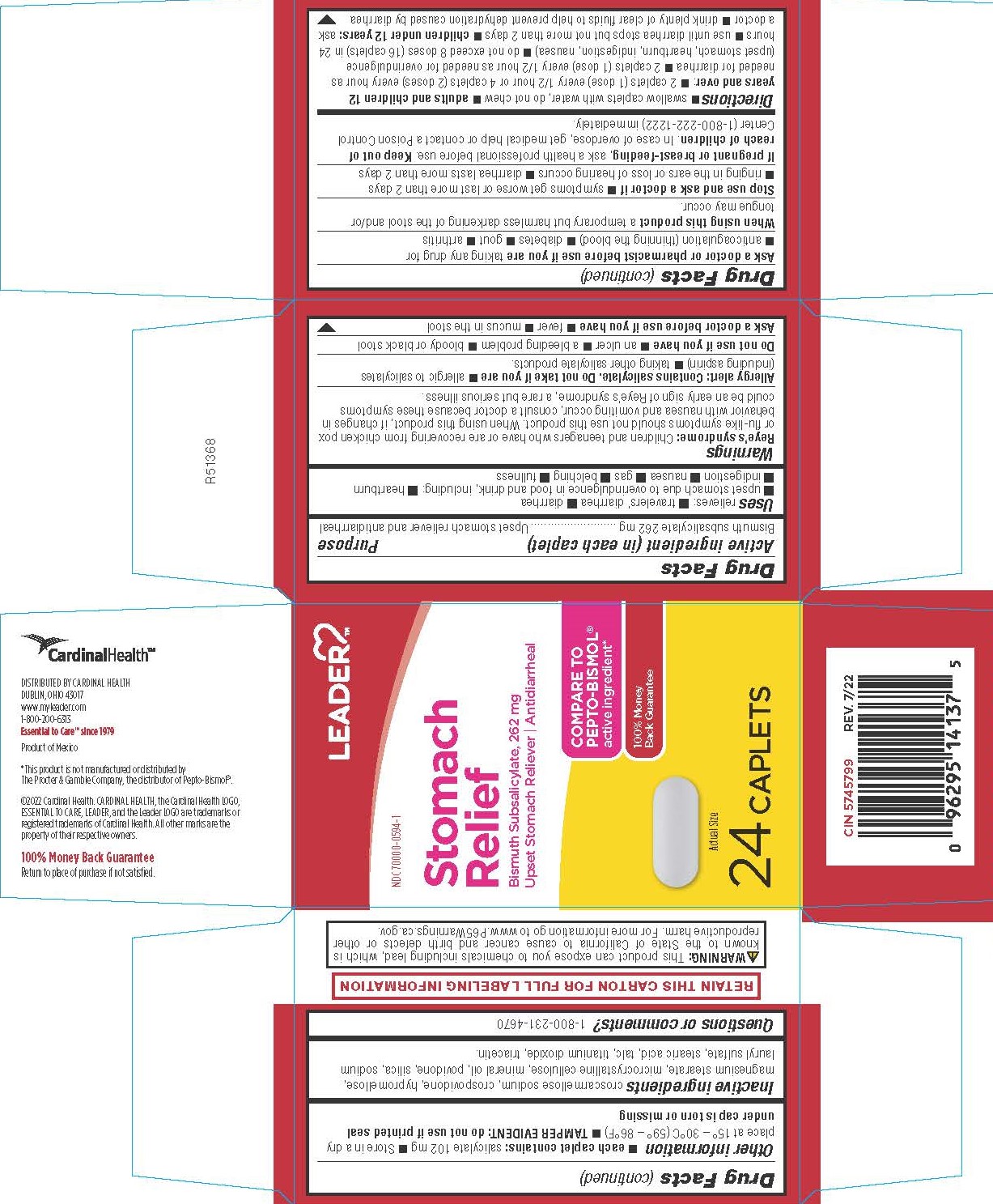
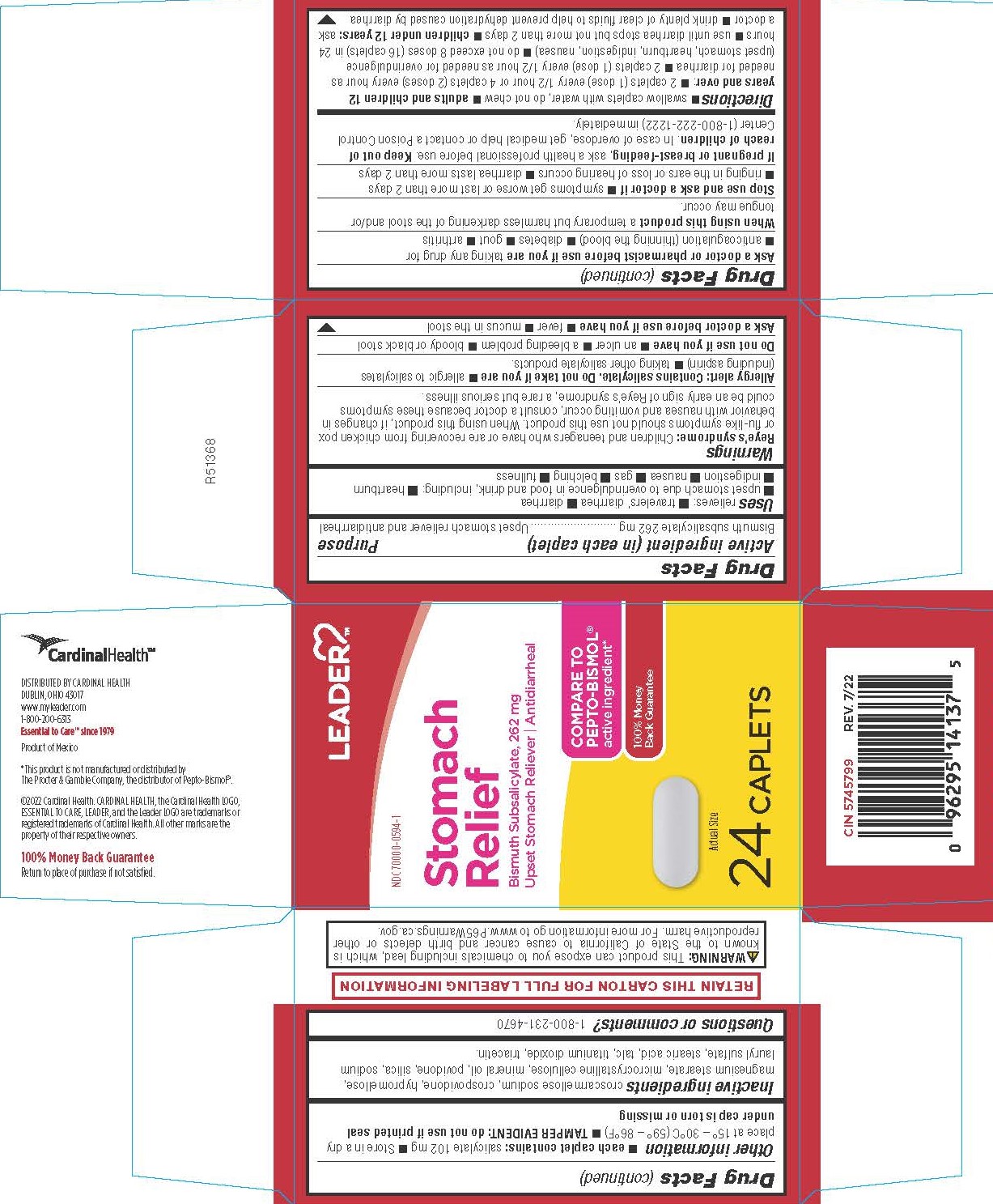
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are
- allergic to salicylates (including aspirin)
- taking other salicylate products.
Ask a doctor or pharmacist before use if you are taking any drug for
- anticoagulation (thinning the blood)
- diabetes
- gout
- arthritis
-
Directions
- swallow caplets with water, do not chew
adults and children 12 years and over:
- 2 caplets (1 dose) every 1/2 hour or 4 caplets (2 doses) every hour as needed for diarrhea
- 2 caplets (1 dose) every 1/2 hour as needed for overindulgence (upset stomach, heartburn, indigestion, nausea)
- do not exceed 8 doses (16 caplets) in 24 hours
- use until diarrhea stops but not more than 2 days
children under 12 years: ask a doctor
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- Other information
- INACTIVE INGREDIENT
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
RETAIN THIS CARTON FOR FULL LABELING INFORMATION
WARNING: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
COMPARE TO PEPTO-BISMOL active ingredient*
*This product is not manufactured or distributed by Procter & Gamble Inc., the distributor of Pepto-Bismol®.
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
www.myleader.com
1-800-200-6313
Essential to Care™ since 1979
Product of Mexico
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BISMUTH SUBSALICYLATE
bismuth subsalicylate tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70000-0594 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISMUTH SUBSALICYLATE (UNII: 62TEY51RR1) (SALICYLIC ACID - UNII:O414PZ4LPZ) BISMUTH SUBSALICYLATE 262 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (UNII: 2S7830E561) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) TALC (UNII: 7SEV7J4R1U) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSES (UNII: 3NXW29V3WO) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) TRIACETIN (UNII: XHX3C3X673) LIGHT MINERAL OIL (UNII: N6K5787QVP) POVIDONE K30 (UNII: U725QWY32X) STEARIC ACID (UNII: 4ELV7Z65AP) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape OVAL Size 17mm Flavor Imprint Code V Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70000-0594-1 1 in 1 CARTON 01/03/2022 1 24 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M008 01/03/2022 Labeler - Cardinal Health (063997360)