Label: ANTIPHLAMINE- l-menthol, l-camphor, methyl salicylate lotion
- NDC Code(s): 72988-0028-1
- Packager: Lydia Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated July 5, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
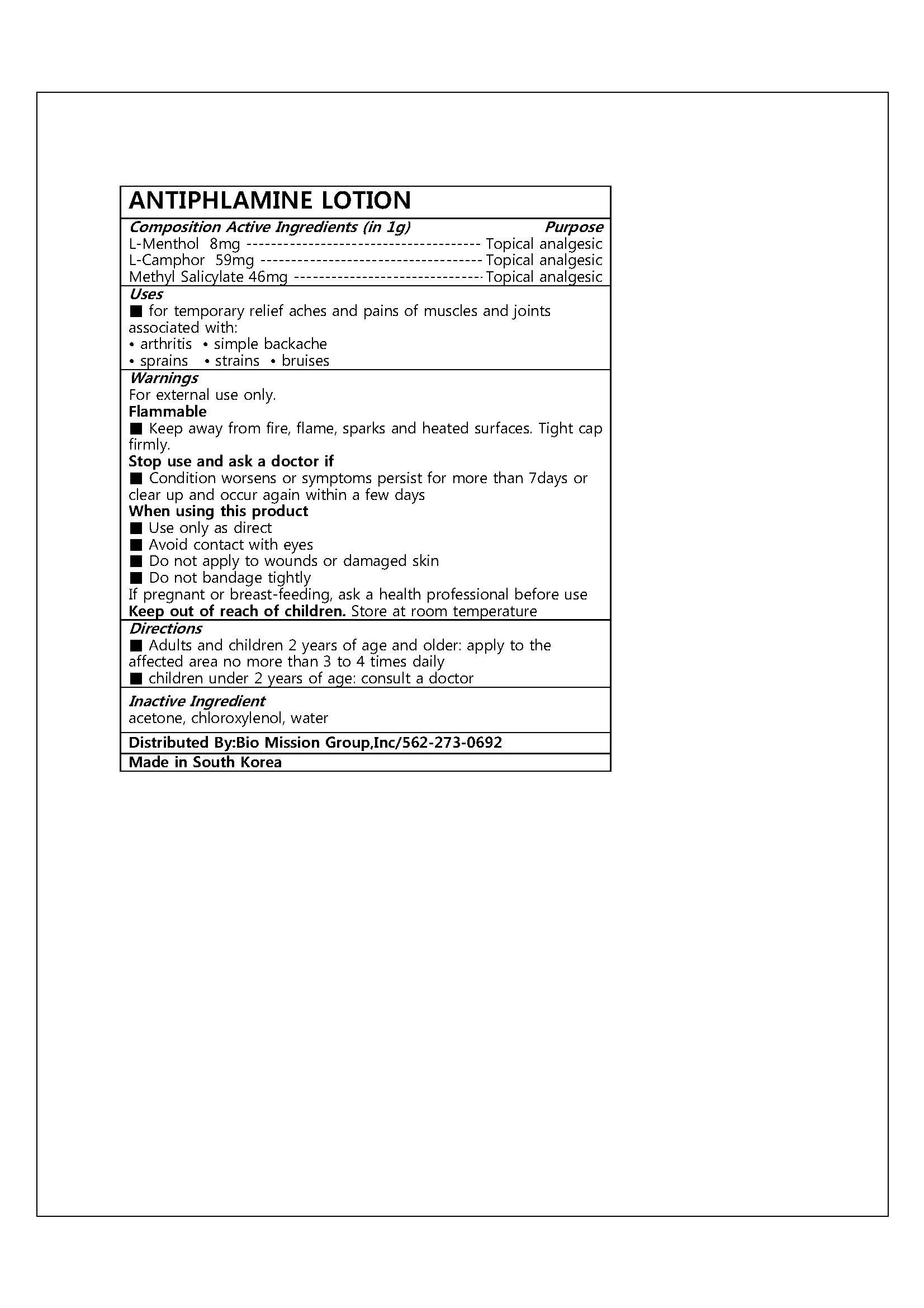
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only.
Flammable
■ Keep away from fire, flame, sparks and heated surfaces. Tight cap firmly.
Stop use and ask a doctor if
■ Condition worsens or symptoms persist for more than 7days or clear up and occur again within a few days
When using this product
■ Use only as direct
■ Avoid contact with eyes
■ Do not apply to wounds or damaged skin
■ Do not bandage tightly
If pregnant or breast-feeding, ask a health professional before use
Keep out of reach of children
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTIPHLAMINE
l-menthol, l-camphor, methyl salicylate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72988-0028 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR, (-)- (UNII: 213N3S8275) (CAMPHOR, (-)- - UNII:213N3S8275) CAMPHOR, (-)- 59 mg in 1 g LEVOMENTHOL (UNII: BZ1R15MTK7) (LEVOMENTHOL - UNII:BZ1R15MTK7) LEVOMENTHOL 8 mg in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 46 mg in 1 g Inactive Ingredients Ingredient Name Strength ACETONE (UNII: 1364PS73AF) CHLOROXYLENOL (UNII: 0F32U78V2Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72988-0028-1 30 g in 1 JAR; Type 0: Not a Combination Product 01/02/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/02/2022 Labeler - Lydia Co., Ltd. (695735569) Registrant - Lydia Co., Ltd. (695735569) Establishment Name Address ID/FEI Business Operations Lydia Co., Ltd. 695735569 manufacture(72988-0028)