VITAFOL CAPLET- vitamin a, ascorbic acid, vitamin d, .alpha.-tocopherol, thiamine mononitrate, riboflavin, niacin, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium, and iron capsule
Exeltis USA, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
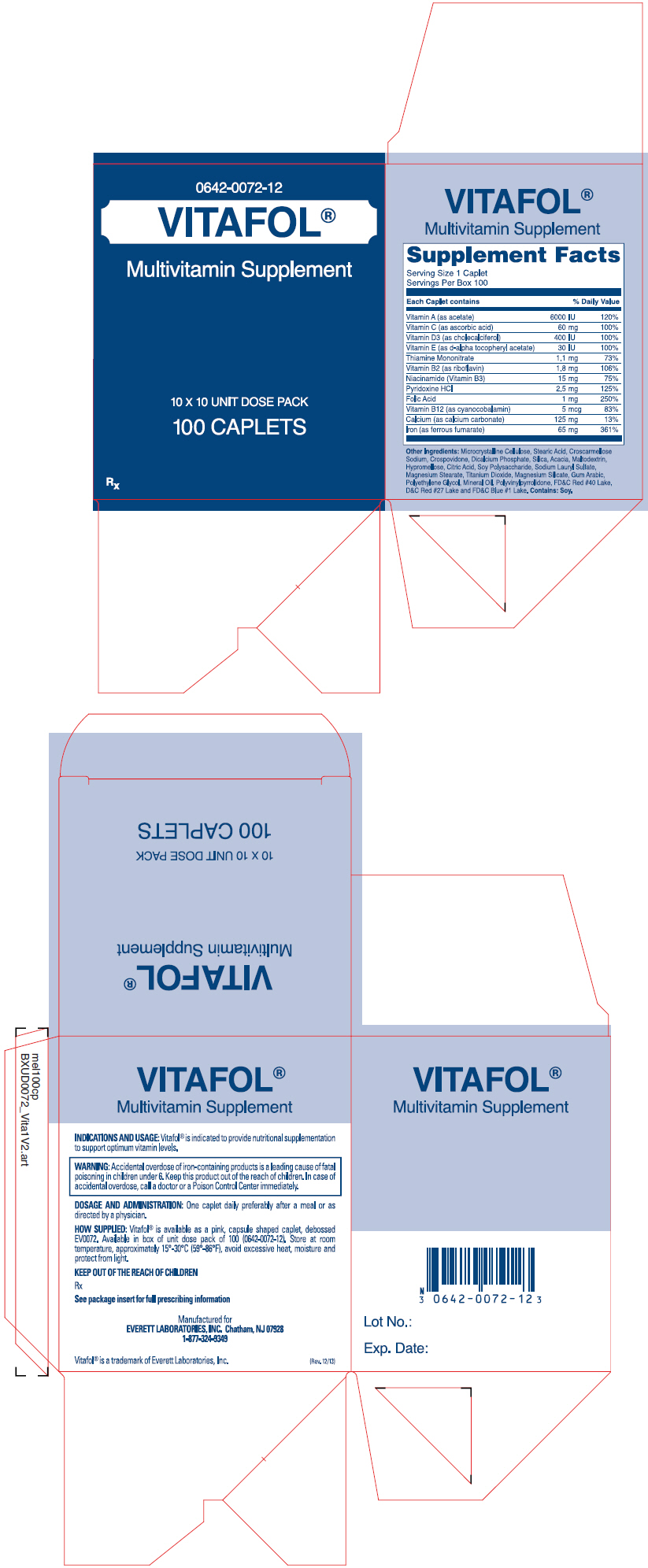
VITAFOL
®
Multivitamin and Mineral Supplement
COMPOSITION
| Each caplet contains: | |
| Vitamin A (as acetate) | 6000 IU |
| Vitamin C (as ascorbic acid) | 60 mg |
| Vitamin D-3 (as cholecalciferol) | 400 IU |
| Vitamin E (as d-alpha tocopheryl acetate) | 30 IU |
| Thiamine Mononitrate | 1.1 mg |
| Vitamin B-2 (as riboflavin) | 1.8 mg |
| Niacinamide (Vitamin B-3) | 15 mg |
| Pyridoxine HCl | 2.5 mg |
| Folic Acid | 1 .0 mg |
| Vitamin B-12 (as cyanocobalamin) | 5 mcg |
| Calcium (as calcium carbonate) | 125 mg |
| Iron (as ferrous fumarate) | 65 mg |
Other Ingredients: Microcrystalline Cellulose, Stearic Acid, Croscarmellose Sodium, Crospovidone, Dicalcium Phosphate, Silica, Acacia, Maltodextrin, Hypromellose, Citric Acid, Soy Polysaccharide, Sodium Lauryl Sulfate, Magnesium Stearate, Titanium Dioxide, Magnesium Silicate, Gum Arabic, Polyethylene Glycol, Mineral Oil, Polyvinylpyrrolidone, FD&C Red #40 Lake, D&C Red #27 Lake and FD&C Blue #1 Lake. Contains Soy.
INDICATIONS AND USAGE
Vitafol ® is indicated to provide vitamin, mineral supplementation to support optimum vitamin levels.
CONTRAINDICATIONS
Vitafol ® is contraindicated in patients with hypersensitivity to any of its components or color additives. Folic acid is contraindicated in patients with untreated and uncomplicated pernicious anemia, and in those with anaphylactic sensitivity to folic acid. Iron therapy is contraindicated in patients with hemochromatosis and patients with iron storage disease or the potential for iron storage disease due to chronic hemolytic anemia (e.g., inherited anomalies of hemoglobin structure or synthesis and/or red cell enzyme deficiencies, etc.); pyridoxine responsive anemia, or cirrhosis of the liver. Cyanocobalamin is contraindicated in patients with sensitivity to cobalt or to cyanocobalamin (Vitamin B-12).
WARNINGS/PRECAUTIONS
Vitamin D supplementation should be used with caution in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones. High doses of vitamin D can lead to elevated levels of calcium that reside in the blood and soft tissues. Bone pain, high blood pressure, formation of kidney stones, renal failure, and increased risk of heart disease can occur. Prolonged therapeutic use of iron salts may produce iron storage disease.
WARNING
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of the reach of children. In case of accidental overdose, call a doctor or a Poison Control Center immediately.
Folic acid, especially in doses above 0.1 mg daily, may obscure pernicious anemia, in that hematologic remission may occur while neurological manifestations remain progressive. The use of folic acid doses above 1 mg daily may precipitate or exacerbate the neurological damage of vitamin B12 deficiency.
Avoid overdosage. Keep out of the reach of children.
Drug Interactions
High doses of folic acid may result in decreased serum levels of the anticonvulsant drugs.
Vitamin D supplementation should not be given with large amounts of calcium in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones.
Consult appropriate references for additional specific vitamin-drug interactions.
ADVERSE REACTIONS
Adverse reactions have been reported with specific vitamins and minerals, but generally at doses substantially higher than those in Vitafol ®.
DOSAGE AND ADMINISTRATION
One caplet daily, preferably after a meal or as prescribed by a physician.
HOW SUPPLIED
Vitafol ® is available as a pink, capsule shaped caplet, debossed EV0072. Available in box of unit dose pack of 100 (0642-0072-12).
| VITAFOL
CAPLET
vitamin a, ascorbic acid, vitamin d, .alpha.-tocopherol, thiamine mononitrate, riboflavin, niacin, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium, and iron capsule |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Exeltis USA, Inc. (071170534) |
| Registrant - Exeltis USA, Inc. (071170534) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Exeltis USA, Inc. | 071170534 | label(0642-0072) | |