Label: VIREAD- tenofovir disoproxil fumarate tablet, coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 69189-0401-1 - Packager: Avera McKennan Hospital
- This is a repackaged label.
- Source NDC Code(s): 61958-0401
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 24, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VIREAD safely and effectively. See full prescribing information for VIREAD.
VIREAD® (tenofovir disoproxil fumarate) tablets, for oral use
VIREAD® (tenofovir disoproxil fumarate) powder, for oral use
Initial U.S. Approval: 2001WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT EXACERBATION OF HEPATITIS
See full prescribing information for complete boxed warning.
- Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including VIREAD. (5.1)
- Severe acute exacerbations of hepatitis have been reported in HBV-infected patients who have discontinued anti-hepatitis B therapy, including VIREAD. Hepatic function should be monitored closely in these patients. If appropriate, resumption of anti-hepatitis B therapy may be warranted. (5.2)
INDICATIONS AND USAGE
VIREAD is a nucleotide analog HIV-1 reverse transcriptase inhibitor and an HBV reverse transcriptase inhibitor.
VIREAD is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients 2 years of age and older. (1)
VIREAD is indicated for the treatment of chronic hepatitis B in adults and pediatric patients 12 years of age and older. (1)
DOSAGE AND ADMINISTRATION
- Recommended dose for the treatment of HIV-1 or chronic hepatitis B in adults and pediatric patients 12 years of age and older (35 kg or more): 300 mg once daily taken orally without regard to food. (2.1)
- Recommended dose for the treatment of HIV-1 in pediatric patients (2 to less than 12 years of age):
Tablets: for pediatric patients weighing greater than or equal to 17 kg who can swallow an intact tablet, one VIREAD tablet (150, 200, 250 or 300 mg based on body weight) once daily taken orally without regard to food. (2.2)
Oral powder: 8 mg/kg VIREAD oral powder (up to a maximum of 300 mg) once daily with food. (2.2) - Dose recommended in renal impairment in adults:
Creatinine clearance 30–49 mL/min: 300 mg every 48 hours. (2.3)
Creatinine clearance 10–29 mL/min: 300 mg every 72 to 96 hours. (2.3)
Hemodialysis: 300 mg every 7 days or after approximately 12 hours of dialysis. (2.3)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- New onset or worsening renal impairment: Can include acute renal failure and Fanconi syndrome. Assess estimated creatinine clearance before initiating treatment with VIREAD. In patients at risk for renal dysfunction, assess estimated creatinine clearance, serum phosphorus, urine glucose and urine protein before initiating treatment with VIREAD and periodically during treatment. Avoid administering VIREAD with concurrent or recent use of nephrotoxic drugs. (5.3)
- Coadministration with Other Products: Do not use with other tenofovir-containing products (e.g., ATRIPLA, COMPLERA, STRIBILD and TRUVADA). Do not administer in combination with HEPSERA. (5.4)
- HIV testing: HIV antibody testing should be offered to all HBV-infected patients before initiating therapy with VIREAD. VIREAD should only be used as part of an appropriate antiretroviral combination regimen in HIV-infected patients with or without HBV coinfection. (5.5)
- Decreases in bone mineral density (BMD): Consider assessment of BMD in patients with a history of pathologic fracture or other risk factors for osteoporosis or bone loss. (5.6)
- Redistribution/accumulation of body fat: Observed in HIV-infected patients receiving antiretroviral combination therapy. (5.7)
- Immune reconstitution syndrome: Observed in HIV-infected patients. May necessitate further evaluation and treatment. (5.8)
- Triple nucleoside-only regimens: Early virologic failure has been reported in HIV-infected patients. Monitor carefully and consider treatment modification. (5.9)
ADVERSE REACTIONS
In HIV-infected adult subjects: Most common adverse reactions (incidence greater than or equal to 10%, Grades 2–4) are rash, diarrhea, headache, pain, depression, asthenia, and nausea. (6.1)
In HBV-infected subjects with compensated liver disease: most common adverse reaction (all grades) was nausea (9%). (6.1)
In pediatric subjects: Adverse reactions in pediatric subjects were consistent with those observed in adults. (6.1)
In HBV-infected subjects with decompensated liver disease: most common adverse reactions (incidence greater than or equal to 10%, all grades) were abdominal pain, nausea, insomnia, pruritus, vomiting, dizziness, and pyrexia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Gilead Sciences, Inc. at 1-800-GILEAD-5 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- Didanosine: Coadministration increases didanosine concentrations. Use with caution and monitor for evidence of didanosine toxicity (e.g., pancreatitis, neuropathy). Consider dose reductions or discontinuations of didanosine if warranted. (7.1)
- HIV-1 protease inhibitors: Coadministration decreases atazanavir concentrations and increases tenofovir concentrations. When coadministered with VIREAD, use atazanavir given with ritonavir. Coadministration of VIREAD with atazanavir and ritonavir, darunavir and ritonavir, or lopinavir/ritonavir increases tenofovir concentrations. Monitor for evidence of tenofovir toxicity. (7.2)
USE IN SPECIFIC POPULATIONS
- Nursing mothers: Women infected with HIV should be instructed not to breast feed. (8.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2015
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT EXACERBATION OF HEPATITIS
1 INDICATIONS AND USAGE
1.1 HIV-1 Infection
1.2 Chronic Hepatitis B
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose in Adults and Pediatric Patients 12 Years of Age and Older (35 kg or more)
2.2 Recommended Dose in Pediatric Patients 2 Years to Less than 12 Years of Age
2.3 Dose Adjustment for Renal Impairment in Adults
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Lactic Acidosis/Severe Hepatomegaly with Steatosis
5.2 Exacerbation of Hepatitis after Discontinuation of Treatment
5.3 New Onset or Worsening Renal Impairment
5.4 Coadministration with Other Products
5.5 Patients Coinfected with HIV-1 and HBV
5.6 Bone Effects
5.7 Fat Redistribution
5.8 Immune Reconstitution Syndrome
5.9 Early Virologic Failure
6 ADVERSE REACTIONS
6.1 Adverse Reactions from Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Didanosine
7.2 HIV-1 Protease Inhibitors
7.3 Drugs Affecting Renal Function
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Impaired Renal Function
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Clinical Efficacy in Adults with HIV-1 Infection
14.2 Clinical Efficacy in Adults with Chronic Hepatitis B
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT EXACERBATION OF HEPATITIS
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including VIREAD, in combination with other antiretrovirals [See Warnings and Precautions (5.1)].
Severe acute exacerbations of hepatitis have been reported in HBV-infected patients who have discontinued anti-hepatitis B therapy, including VIREAD. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue anti-hepatitis B therapy, including VIREAD. If appropriate, resumption of anti-hepatitis B therapy may be warranted [See Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGE
1.1 HIV-1 Infection
VIREAD is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients 2 years of age and older.
The following points should be considered when initiating therapy with VIREAD for the treatment of HIV-1 infection:
- VIREAD should not be used in combination with ATRIPLA®, COMPLERA®, STRIBILD®, or TRUVADA® [See Warnings and Precautions (5.4)].
1.2 Chronic Hepatitis B
VIREAD is indicated for the treatment of chronic hepatitis B in adults and pediatric patients 12 years of age and older.
The following points should be considered when initiating therapy with VIREAD for the treatment of HBV infection:
- The indication in adults is based on safety and efficacy data from treatment of subjects who were nucleoside-treatment-naïve and subjects who were treatment-experienced with documented resistance to lamivudine. Subjects were adults with HBeAg-positive and HBeAg-negative chronic hepatitis B with compensated liver disease [See Clinical Studies (14.2)].
- VIREAD was evaluated in a limited number of subjects with chronic hepatitis B and decompensated liver disease [See Adverse Reactions (6.1), Clinical Studies (14.2)].
- The numbers of subjects in clinical trials who had adefovir resistance-associated substitutions at baseline were too small to reach conclusions of efficacy [See Microbiology (12.4), Clinical Studies (14.2)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose in Adults and Pediatric Patients 12 Years of Age and Older (35 kg or more)
For the treatment of HIV-1 or chronic hepatitis B: The dose is one 300 mg VIREAD tablet once daily taken orally, without regard to food.
For patients unable to swallow VIREAD tablets, the oral powder formulation (7.5 scoops) may be used.
In the treatment of chronic hepatitis B, the optimal duration of treatment is unknown. Safety and efficacy in pediatric patients with chronic hepatitis B weighing less than 35 kg have not been established.
2.2 Recommended Dose in Pediatric Patients 2 Years to Less than 12 Years of Age
HIV-1 Infection
For the treatment of HIV-1 in pediatric patients 2 years of age and older, the recommended oral dose of VIREAD is 8 mg of tenofovir disoproxil fumarate per kilogram of body weight (up to a maximum of 300 mg) once daily administered as oral powder or tablets.
VIREAD oral powder should be measured only with the supplied dosing scoop. One level scoop delivers 1 g of powder which contains 40 mg of tenofovir disoproxil fumarate. VIREAD oral powder should be mixed in a container with 2 to 4 ounces of soft food not requiring chewing (e.g., applesauce, baby food, yogurt). The entire mixture should be ingested immediately to avoid a bitter taste. Do not administer VIREAD oral powder in a liquid as the powder may float on top of the liquid even after stirring. Further patient instructions on how to administer VIREAD oral powder with the supplied dosing scoop are provided in the FDA-approved patient labeling (Patient Information).
VIREAD is also available as tablets in 150, 200, 250 and 300 mg strengths for pediatric patients who weigh greater than or equal to 17 kg and who are able to reliably swallow intact tablets. The dose is one tablet once daily taken orally, without regard to food.
Tables 1 and 2 contain dosing recommendations for VIREAD oral powder and tablets based on body weight. Weight should be monitored periodically and the VIREAD dose adjusted accordingly.
Table 1 Dosing Recommendations for Pediatric Patients ≥2 Years of Age Using VIREAD Oral Powder Body Weight
Kilogram (kg)Oral Powder Once Daily
Scoops of Powder10 to <12 2 12 to <14 2.5 14 to <17 3 17 to <19 3.5 19 to <22 4 22 to <24 4.5 24 to <27 5 27 to <29 5.5 29 to <32 6 32 to <34 6.5 34 to <35 7 ≥35 7.5 Table 2 Dosing Recommendations for Pediatric Patients ≥2 Years of Age and Weighing ≥17 kg Using VIREAD Tablets Body Weight
Kilogram (kg)Tablets Once Daily 17 to <22 150 mg 22 to <28 200 mg 28 to <35 250 mg ≥35 300 mg 2.3 Dose Adjustment for Renal Impairment in Adults
Significantly increased drug exposures occurred when VIREAD was administered to subjects with moderate to severe renal impairment [See Clinical Pharmacology (12.3)]. Therefore, the dosing interval of VIREAD tablets 300 mg should be adjusted in patients with baseline creatinine clearance below 50 mL/min using the recommendations in Table 3. These dosing interval recommendations are based on modeling of single-dose pharmacokinetic data in non-HIV and non-HBV infected subjects with varying degrees of renal impairment, including end-stage renal disease requiring hemodialysis. The safety and effectiveness of these dosing interval adjustment recommendations have not been clinically evaluated in patients with moderate or severe renal impairment, therefore clinical response to treatment and renal function should be closely monitored in these patients [See Warnings and Precautions (5.3)]. There are no data to recommend use of VIREAD tablets 150, 200 or 250 mg or VIREAD oral powder in patients with renal impairment.
No dose adjustment of VIREAD tablets 300 mg is necessary for patients with mild renal impairment (creatinine clearance 50–80 mL/min). Routine monitoring of estimated creatinine clearance, serum phosphorus, urine glucose, and urine protein should be performed in patients with mild renal impairment [See Warnings and Precautions (5.3)].
Table 3 Dosage Adjustment for Patients with Altered Creatinine Clearance Creatinine Clearance
(mL/min)*≥50 30–49 10–29 Hemodialysis Patients Recommended 300 mg Dosing Interval Every 24 hours Every 48 hours Every 72 to 96 hours Every 7 days or after a total of approximately 12 hours of dialysis† The pharmacokinetics of tenofovir have not been evaluated in non-hemodialysis patients with creatinine clearance below 10 mL/min; therefore, no dosing recommendation is available for these patients.
No data are available to make dose recommendations in pediatric patients with renal impairment.
-
3 DOSAGE FORMS AND STRENGTHS
VIREAD is available as tablets or as an oral powder.
VIREAD tablets 150 mg contain 150 mg of tenofovir disoproxil fumarate, which is equivalent to 123 mg of tenofovir disoproxil. The tablets are triangle-shaped, white, film-coated, and debossed with "GSI" on one side and "150" on the other side.
VIREAD tablets 200 mg contain 200 mg of tenofovir disoproxil fumarate, which is equivalent to 163 mg of tenofovir disoproxil. The tablets are round-shaped, white, film-coated, and debossed with "GSI" on one side and "200" on the other side.
VIREAD tablets 250 mg contain 250 mg of tenofovir disoproxil fumarate, which is equivalent to 204 mg of tenofovir disoproxil. The tablets are capsule-shaped, white, film-coated, and debossed with "GSI" on one side and "250" on the other side.
VIREAD tablets 300 mg contain 300 mg of tenofovir disoproxil fumarate, which is equivalent to 245 mg of tenofovir disoproxil. The tablets are almond-shaped, light blue, film-coated, and debossed with "GILEAD" and "4331" on one side and with "300" on the other side.
The oral powder consists of white, taste-masked, coated granules containing 40 mg of tenofovir disoproxil fumarate, which is equivalent to 33 mg of tenofovir disoproxil, per level scoop. Each level scoop contains 1 gram of oral powder.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including VIREAD, in combination with other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Particular caution should be exercised when administering nucleoside analogs to any patient with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with VIREAD should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
5.2 Exacerbation of Hepatitis after Discontinuation of Treatment
Discontinuation of anti-HBV therapy, including VIREAD, may be associated with severe acute exacerbations of hepatitis. Patients infected with HBV who discontinue VIREAD should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment. If appropriate, resumption of anti-hepatitis B therapy may be warranted.
5.3 New Onset or Worsening Renal Impairment
Tenofovir is principally eliminated by the kidney. Renal impairment, including cases of acute renal failure and Fanconi syndrome (renal tubular injury with severe hypophosphatemia), has been reported with the use of VIREAD [See Adverse Reactions (6.2)].
It is recommended that estimated creatinine clearance be assessed in all patients prior to initiating therapy and as clinically appropriate during therapy with VIREAD. In patients at risk of renal dysfunction, including patients who have previously experienced renal events while receiving HEPSERA®, it is recommended that estimated creatinine clearance, serum phosphorus, urine glucose, and urine protein be assessed prior to initiation of VIREAD, and periodically during VIREAD therapy.
Dosing interval adjustment of VIREAD and close monitoring of renal function are recommended in all patients with creatinine clearance below 50 mL/min [See Dosage and Administration (2.3)]. No safety or efficacy data are available in patients with renal impairment who received VIREAD using these dosing guidelines, so the potential benefit of VIREAD therapy should be assessed against the potential risk of renal toxicity.
VIREAD should be avoided with concurrent or recent use of a nephrotoxic agent (e.g., high-dose or multiple non-steroidal anti-inflammatory drugs (NSAIDs)) [See Drug Interactions (7.3)]. Cases of acute renal failure after initiation of high dose or multiple NSAIDs have been reported in HIV-infected patients with risk factors for renal dysfunction who appeared stable on tenofovir DF. Some patients required hospitalization and renal replacement therapy. Alternatives to NSAIDs should be considered, if needed, in patients at risk for renal dysfunction.
Persistent or worsening bone pain, pain in extremities, fractures and/or muscular pain or weakness may be manifestations of proximal renal tubulopathy and should prompt an evaluation of renal function in at-risk patients.
5.4 Coadministration with Other Products
VIREAD should not be used in combination with the fixed-dose combination products ATRIPLA, COMPLERA, STRIBILD, or TRUVADA since tenofovir disoproxil fumarate is a component of these products.
VIREAD should not be administered in combination with HEPSERA (adefovir dipivoxil) [See Drug Interactions (7.3)].
5.5 Patients Coinfected with HIV-1 and HBV
Due to the risk of development of HIV-1 resistance, VIREAD should only be used in HIV-1 and HBV coinfected patients as part of an appropriate antiretroviral combination regimen.
HIV-1 antibody testing should be offered to all HBV-infected patients before initiating therapy with VIREAD. It is also recommended that all patients with HIV-1 be tested for the presence of chronic hepatitis B before initiating treatment with VIREAD.
5.6 Bone Effects
Bone Mineral Density:
In clinical trials in HIV-1 infected adults, VIREAD was associated with slightly greater decreases in bone mineral density (BMD) and increases in biochemical markers of bone metabolism, suggesting increased bone turnover relative to comparators. Serum parathyroid hormone levels and 1,25 Vitamin D levels were also higher in subjects receiving VIREAD [See Adverse Reactions (6.1)].
Clinical trials evaluating VIREAD in pediatric and adolescent subjects were conducted. Under normal circumstances, BMD increases rapidly in pediatric patients. In HIV-1 infected subjects aged 2 years to less than 18 years, bone effects were similar to those observed in adult subjects and suggest increased bone turnover. Total body BMD gain was less in the VIREAD-treated HIV-1 infected pediatric subjects as compared to the control groups. Similar trends were observed in chronic hepatitis B infected adolescent subjects aged 12 years to less than 18 years. In all pediatric trials, skeletal growth (height) appeared to be unaffected [See Adverse Reactions (6.1)].
The effects of VIREAD-associated changes in BMD and biochemical markers on long-term bone health and future fracture risk are unknown. Assessment of BMD should be considered for adults and pediatric patients who have a history of pathologic bone fracture or other risk factors for osteoporosis or bone loss. Although the effect of supplementation with calcium and vitamin D was not studied, such supplementation may be beneficial for all patients. If bone abnormalities are suspected then appropriate consultation should be obtained.
Mineralization Defects:
Cases of osteomalacia associated with proximal renal tubulopathy, manifested as bone pain or pain in extremities and which may contribute to fractures, have been reported in association with the use of VIREAD [See Adverse Reactions (6.2)]. Arthralgias and muscle pain or weakness have also been reported in cases of proximal renal tubulopathy. Hypophosphatemia and osteomalacia secondary to proximal renal tubulopathy should be considered in patients at risk of renal dysfunction who present with persistent or worsening bone or muscle symptoms while receiving products containing tenofovir DF [See Warnings and Precautions (5.3)].
5.7 Fat Redistribution
In HIV-infected patients redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving combination antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
5.8 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in HIV-infected patients treated with combination antiretroviral therapy, including VIREAD. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections [such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis], which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution, however, the time to onset is more variable, and can occur many months after initiation of treatment.
5.9 Early Virologic Failure
Clinical trials in HIV-infected subjects have demonstrated that certain regimens that only contain three nucleoside reverse transcriptase inhibitors (NRTI) are generally less effective than triple drug regimens containing two NRTIs in combination with either a non-nucleoside reverse transcriptase inhibitor or a HIV-1 protease inhibitor. In particular, early virological failure and high rates of resistance substitutions have been reported. Triple nucleoside regimens should therefore be used with caution. Patients on a therapy utilizing a triple nucleoside-only regimen should be carefully monitored and considered for treatment modification.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in other sections of the labeling:
- Lactic Acidosis/Severe Hepatomegaly with Steatosis [See Boxed Warning, Warnings and Precautions (5.1)].
- Severe Acute Exacerbation of Hepatitis [See Boxed Warning, Warnings and Precautions (5.2)].
- New Onset or Worsening Renal Impairment [See Warnings and Precautions (5.3)].
- Bone Effects [See Warnings and Precautions (5.6)].
- Immune Reconstitution Syndrome [See Warnings and Precautions (5.8)].
6.1 Adverse Reactions from Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials in Adult Patients with HIV-1 Infection
More than 12,000 subjects have been treated with VIREAD alone or in combination with other antiretroviral medicinal products for periods of 28 days to 215 weeks in clinical trials and expanded access programs. A total of 1,544 subjects have received VIREAD 300 mg once daily in clinical trials; over 11,000 subjects have received VIREAD in expanded access programs.
The most common adverse reactions (incidence greater than or equal to 10%, Grades 2–4) identified from any of the 3 large controlled clinical trials include rash, diarrhea, headache, pain, depression, asthenia, and nausea.
Treatment-Naïve Patients
Study 903 - Treatment-Emergent Adverse Reactions: The most common adverse reactions seen in a double-blind comparative controlled trial in which 600 treatment-naïve subjects received VIREAD (N=299) or stavudine (N=301) in combination with lamivudine and efavirenz for 144 weeks (Study 903) were mild to moderate gastrointestinal events and dizziness.
Mild adverse reactions (Grade 1) were common with a similar incidence in both arms, and included dizziness, diarrhea, and nausea. Selected treatment-emergent moderate to severe adverse reactions are summarized in Table 4.
Table 4 Selected Treatment-Emergent Adverse Reactions* (Grades 2–4) Reported in ≥5% in Any Treatment Group in Study 903 (0–144 Weeks) VIREAD + 3TC + EFV d4T + 3TC + EFV N=299 N=301 - *
- Frequencies of adverse reactions are based on all treatment-emergent adverse events, regardless of relationship to study drug.
- †
- Lipodystrophy represents a variety of investigator-described adverse events not a protocol-defined syndrome.
- ‡
- Peripheral neuropathy includes peripheral neuritis and neuropathy.
- §
- Rash event includes rash, pruritus, maculopapular rash, urticaria, vesiculobullous rash, and pustular rash.
Body as a Whole Headache 14% 17% Pain 13% 12% Fever 8% 7% Abdominal pain 7% 12% Back pain 9% 8% Asthenia 6% 7% Digestive System Diarrhea 11% 13% Nausea 8% 9% Dyspepsia 4% 5% Vomiting 5% 9% Metabolic Disorders Lipodystrophy† 1% 8% Musculoskeletal Arthralgia 5% 7% Myalgia 3% 5% Nervous System Depression 11% 10% Insomnia 5% 8% Dizziness 3% 6% Peripheral neuropathy‡ 1% 5% Anxiety 6% 6% Respiratory Pneumonia 5% 5% Skin and Appendages Rash event§ 18% 12% Laboratory Abnormalities: With the exception of fasting cholesterol and fasting triglyceride elevations that were more common in the stavudine group (40% and 9%) compared with VIREAD (19% and 1%) respectively, laboratory abnormalities observed in this trial occurred with similar frequency in the VIREAD and stavudine treatment arms. A summary of Grades 3–4 laboratory abnormalities is provided in Table 5.
Table 5 Grades 3–4 Laboratory Abnormalities Reported in ≥1% of VIREAD-Treated Subjects in Study 903 (0–-144 Weeks) VIREAD + 3TC + EFV d4T + 3TC + EFV N=299 N=301 Any ≥ Grade 3 Laboratory Abnormality 36% 42% Fasting Cholesterol (>240 mg/dL) 19% 40% Creatine Kinase (M: >990 U/L; F: >845 U/L) 12% 12% Serum Amylase (>175 U/L) 9% 8% AST (M: >180 U/L; F: >170 U/L) 5% 7% ALT (M: >215 U/L; F: >170 U/L) 4% 5% Hematuria (>100 RBC/HPF) 7% 7% Neutrophils (<750/mm3) 3% 1% Fasting Triglycerides (>750 mg/dL) 1% 9% Study 934 – Treatment-Emergent Adverse Reactions: In Study 934, 511 antiretroviral-naïve subjects received either VIREAD + EMTRIVA® administered in combination with efavirenz (N=257) or zidovudine/lamivudine administered in combination with efavirenz (N=254). Adverse reactions observed in this trial were generally consistent with those seen in previous studies in treatment-experienced or treatment-naïve subjects (Table 6).
Changes in Bone Mineral Density:
In HIV-1 infected adult subjects in Study 903, there was a significantly greater mean percentage decrease from baseline in BMD at the lumbar spine in subjects receiving VIREAD + lamivudine + efavirenz (-2.2% ± 3.9) compared with subjects receiving stavudine + lamivudine + efavirenz (-1.0% ± 4.6) through 144 weeks. Changes in BMD at the hip were similar between the two treatment groups (-2.8% ± 3.5 in the VIREAD group vs. -2.4% ± 4.5 in the stavudine group). In both groups, the majority of the reduction in BMD occurred in the first 24–48 weeks of the trial and this reduction was sustained through Week 144. Twenty-eight percent of VIREAD-treated subjects vs. 21% of the stavudine-treated subjects lost at least 5% of BMD at the spine or 7% of BMD at the hip. Clinically relevant fractures (excluding fingers and toes) were reported in 4 subjects in the VIREAD group and 6 subjects in the stavudine group. In addition, there were significant increases in biochemical markers of bone metabolism (serum bone-specific alkaline phosphatase, serum osteocalcin, serum C telopeptide, and urinary N telopeptide) and higher serum parathyroid hormone levels and 1,25 Vitamin D levels in the VIREAD group relative to the stavudine group; however, except for bone-specific alkaline phosphatase, these changes resulted in values that remained within the normal range [See Warnings and Precautions (5.6)].
Table 6 Selected Treatment-Emergent Adverse Reactions* (Grades 2–4) Reported in ≥5% in Any Treatment Group in Study 934 (0–144 Weeks) VIREAD† + FTC + EFV AZT/3TC + EFV N=257 N=254 - *
- Frequencies of adverse reactions are based on all treatment-emergent adverse events, regardless of relationship to study drug.
- †
- From Weeks 96 to 144 of the trial, subjects received TRUVADA with efavirenz in place of VIREAD + EMTRIVA with efavirenz.
- ‡
- Rash event includes rash, exfoliative rash, rash generalized, rash macular, rash maculopapular, rash pruritic, and rash vesicular.
Gastrointestinal Disorder Diarrhea 9% 5% Nausea 9% 7% Vomiting 2% 5% General Disorders and Administration Site Condition Fatigue 9% 8% Infections and Infestations Sinusitis 8% 4% Upper respiratory tract infections 8% 5% Nasopharyngitis 5% 3% Nervous System Disorders Headache 6% 5% Dizziness 8% 7% Psychiatric Disorders Depression 9% 7% Insomnia 5% 7% Skin and Subcutaneous Tissue Disorders Rash event‡ 7% 9% Laboratory Abnormalities: Laboratory abnormalities observed in this trial were generally consistent with those seen in previous trials (Table 7).
Table 7 Significant Laboratory Abnormalities Reported in ≥1% of Subjects in Any Treatment Group in Study 934 (0–144 Weeks) VIREAD* + FTC + EFV AZT/3TC + EFV N=257 N=254 - *
- From Weeks 96 to 144 of the trial, subjects received TRUVADA with efavirenz in place of VIREAD + EMTRIVA with efavirenz.
Any ≥ Grade 3 Laboratory Abnormality 30% 26% Fasting Cholesterol (>240 mg/dL) 22% 24% Creatine Kinase (M: >990 U/L; F: >845 U/L) 9% 7% Serum Amylase (>175 U/L) 8% 4% Alkaline Phosphatase (>550 U/L) 1% 0% AST (M: >180 U/L; F: >170 U/L) 3% 3% ALT (M: >215 U/L; F: >170 U/L) 2% 3% Hemoglobin (<8.0 mg/dL) 0% 4% Hyperglycemia (>250 mg/dL) 2% 1% Hematuria (>75 RBC/HPF) 3% 2% Glycosuria (≥3+) <1% 1% Neutrophils (<750/mm3) 3% 5% Fasting Triglycerides (>750 mg/dL) 4% 2% Treatment-Experienced Patients
Treatment-Emergent Adverse Reactions: The adverse reactions seen in treatment-experienced subjects were generally consistent with those seen in treatment-naïve subjects including mild to moderate gastrointestinal events, such as nausea, diarrhea, vomiting, and flatulence. Less than 1% of subjects discontinued participation in the clinical trials due to gastrointestinal adverse reactions (Study 907).
A summary of moderate to severe, treatment-emergent adverse reactions that occurred during the first 48 weeks of Study 907 is provided in Table 8.
Table 8 Selected Treatment-Emergent Adverse Reactions* (Grades 2–4) Reported in ≥3% in Any Treatment Group in Study 907 (0–48 Weeks) VIREAD
(N=368)
(Week 0–24)Placebo
(N=182)
(Week 0–24)VIREAD
(N=368)
(Week 0–48)Placebo Crossover to VIREAD
(N=170)
(Week 24–48)- *
- Frequencies of adverse reactions are based on all treatment-emergent adverse events, regardless of relationship to study drug.
- †
- Peripheral neuropathy includes peripheral neuritis and neuropathy.
- ‡
- Rash event includes rash, pruritus, maculopapular rash, urticaria, vesiculobullous rash, and pustular rash.
Body as a Whole Asthenia 7% 6% 11% 1% Pain 7% 7% 12% 4% Headache 5% 5% 8% 2% Abdominal pain 4% 3% 7% 6% Back pain 3% 3% 4% 2% Chest pain 3% 1% 3% 2% Fever 2% 2% 4% 2% Digestive System Diarrhea 11% 10% 16% 11% Nausea 8% 5% 11% 7% Vomiting 4% 1% 7% 5% Anorexia 3% 2% 4% 1% Dyspepsia 3% 2% 4% 2% Flatulence 3% 1% 4% 1% Respiratory Pneumonia 2% 0% 3% 2% Nervous System Depression 4% 3% 8% 4% Insomnia 3% 2% 4% 4% Peripheral neuropathy† 3% 3% 5% 2% Dizziness 1% 3% 3% 1% Skin and Appendage Rash event‡ 5% 4% 7% 1% Sweating 3% 2% 3% 1% Musculoskeletal Myalgia 3% 3% 4% 1% Metabolic Weight loss 2% 1% 4% 2% Laboratory Abnormalities: Laboratory abnormalities observed in this trial occurred with similar frequency in the VIREAD and placebo-treated groups. A summary of Grades 3–4 laboratory abnormalities is provided in Table 9.
Table 9 Grades 3–4 Laboratory Abnormalities Reported in ≥1% of VIREAD-Treated Subjects in Study 907 (0–48 Weeks) VIREAD
(N=368)
(Week 0–24)Placebo
(N=182)
(Week 0–24)VIREAD
(N=368)
(Week 0–48)Placebo Crossover to VIREAD
(N=170)
(Week 24–48)Any ≥ Grade 3 Laboratory Abnormality 25% 38% 35% 34% Triglycerides (>750 mg/dL) 8% 13% 11% 9% Creatine Kinase
(M: >990 U/L; F: >845 U/L)7% 14% 12% 12% Serum Amylase (>175 U/L) 6% 7% 7% 6% Glycosuria (≥3+) 3% 3% 3% 2% AST (M: >180 U/L; F: >170 U/L) 3% 3% 4% 5% ALT (M: >215 U/L; F: >170 U/L) 2% 2% 4% 5% Serum Glucose (>250 U/L) 2% 4% 3% 3% Neutrophils (<750/mm3) 1% 1% 2% 1% Clinical Trials in Pediatric Subjects 2 Years of Age and Older with HIV-1 Infection
Assessment of adverse reactions is based on two randomized trials (Studies 352 and 321) in 184 HIV-1 infected pediatric subjects (2 to less than 18 years of age) who received treatment with VIREAD (N=93) or placebo/active comparator (N=91) in combination with other antiretroviral agents for 48 weeks. The adverse reactions observed in subjects who received treatment with VIREAD were consistent with those observed in clinical trials in adults.
Eighty-nine pediatric subjects (2 to less than 12 years of age) received VIREAD in Study 352 for a median exposure of 104 weeks. Of these, 4 subjects discontinued from the trial due to adverse reactions consistent with proximal renal tubulopathy. Three of these 4 subjects presented with hypophosphatemia and also had decreases in total body or spine BMD Z score [See Warnings and Precautions (5.6)].
Changes in Bone Mineral Density:
Clinical trials in HIV-1 infected children and adolescents evaluated BMD changes. In Study 321 (12 to less than 18 years), the mean rate of BMD gain at Week 48 was less in the VIREAD compared to the placebo treatment group. Six VIREAD treated subjects and one placebo treated subject had significant (greater than 4%) lumbar spine BMD loss at Week 48. Changes from baseline BMD Z-scores were –0.341 for lumbar spine and –0.458 for total body in the 28 subjects who were treated with VIREAD for 96 weeks. In Study 352 (2 to less than 12 years), the mean rate of BMD gain in lumbar spine at Week 48 was similar between the VIREAD and the d4T or AZT treatment groups. Total body BMD gain was less in the VIREAD compared to the d4T or AZT treatment groups. One VIREAD-treated subject and none of the d4T or AZT-treated subjects experienced significant (greater than 4%) lumbar spine BMD loss at Week 48. Changes from baseline in BMD Z scores were –0.012 for lumbar spine and –0.338 for total body in the 64 subjects who were treated with VIREAD for 96 weeks. In both trials, skeletal growth (height) appeared to be unaffected [See Warnings and Precautions (5.6)].
Clinical Trials in Adult Subjects with Chronic Hepatitis B and Compensated Liver Disease
Treatment-Emergent Adverse Reactions: In controlled clinical trials in 641 subjects with chronic hepatitis B (0102 and 0103), more subjects treated with VIREAD during the 48-week double-blind period experienced nausea: 9% with VIREAD versus 2% with HEPSERA. Other treatment-emergent adverse reactions reported in more than 5% of subjects treated with VIREAD included: abdominal pain, diarrhea, headache, dizziness, fatigue, nasopharyngitis, back pain and skin rash.
During the open-label phase of treatment with VIREAD (weeks 48–384) in Studies 0102 and 0103, 2% of subjects (13/585) experienced a confirmed increase in serum creatinine of 0.5 mg/dL from baseline. No significant change in the tolerability profile was observed with continued treatment for up to 384 weeks.
Laboratory Abnormalities: A summary of Grades 3–4 laboratory abnormalities through Week 48 is provided in Table 10. Grades 3–4 laboratory abnormalities were similar in subjects continuing VIREAD treatment for up to 384 weeks in these trials.
Table 10 Grades 3–4 Laboratory Abnormalities Reported in ≥1% of VIREAD-Treated Subjects in Studies 0102 and 0103 (0–48 Weeks) VIREAD
(N=426)HEPSERA
(N=215)Any ≥ Grade 3 Laboratory Abnormality 19% 13% Creatine Kinase (M: >990 U/L; F: >845 U/L) 2% 3% Serum Amylase (>175 U/L) 4% 1% Glycosuria (≥3+) 3% <1% AST (M: >180 U/L; F: >170 U/L) 4% 4% ALT (M: >215 U/L; F: >170 U/L) 10% 6% The overall incidence of on-treatment ALT flares (defined as serum ALT greater than 2 × baseline and greater than 10 × ULN, with or without associated symptoms) was similar between VIREAD (2.6%) and HEPSERA (2%). ALT flares generally occurred within the first 4–8 weeks of treatment and were accompanied by decreases in HBV DNA levels. No subject had evidence of decompensation. ALT flares typically resolved within 4 to 8 weeks without changes in study medication.
The adverse reactions observed in subjects with chronic hepatitis B and lamivudine resistance who received treatment with VIREAD were consistent with those observed in other hepatitis B clinical trials in adults.
Clinical Trials in Adult Subjects with Chronic Hepatitis B and Decompensated Liver Disease
In a small randomized, double-blind, active-controlled trial (0108), subjects with CHB and decompensated liver disease received treatment with VIREAD or other antiviral drugs for up to 48 weeks [See Clinical Studies (14.2)]. Among the 45 subjects receiving VIREAD, the most frequently reported treatment-emergent adverse reactions of any severity were abdominal pain (22%), nausea (20%), insomnia (18%), pruritus (16%), vomiting (13%), dizziness (13%), and pyrexia (11%). Two of 45 (4%) subjects died through Week 48 of the trial due to progression of liver disease. Three of 45 (7%) subjects discontinued treatment due to an adverse event. Four of 45 (9%) subjects experienced a confirmed increase in serum creatinine of 0.5 mg/dL (1 subject also had a confirmed serum phosphorus less than 2 mg/dL through Week 48). Three of these subjects (each of whom had a Child-Pugh score greater than or equal to 10 and MELD score greater than or equal to 14 at entry) developed renal failure. Because both VIREAD and decompensated liver disease may have an impact on renal function, the contribution of VIREAD to renal impairment in this population is difficult to ascertain.
One of 45 subjects experienced an on-treatment hepatic flare during the 48 Week trial.
Clinical Trials in Pediatric Subjects 12 Years of Age and Older with Chronic Hepatitis B
Assessment of adverse reactions is based on one randomized study (Study GS-US-174-0115) in 106 pediatric subjects (12 to less than 18 years of age) infected with chronic hepatitis B receiving treatment with VIREAD (N = 52) or placebo (N = 54) for 72 weeks. The adverse reactions observed in pediatric subjects who received treatment with VIREAD were consistent with those observed in clinical trials of VIREAD in adults.
In this study, both the VIREAD and placebo treatment arms experienced an overall increase in mean lumbar spine BMD over 72 weeks, as expected for an adolescent population. The BMD gains from baseline to Week 72 in lumbar spine and total body BMD in VIREAD-treated subjects (+5% and +3%, respectively) were less than the BMD gains observed in placebo-treated subjects (+8% and +5%, respectively). Three subjects in the VIREAD group and two subjects in the placebo group had significant (greater than 4%) lumbar spine BMD loss at Week 72. At baseline, mean BMD Z-scores in subjects randomized to VIREAD were −0.43 for lumbar spine and −0.20 for total body, and mean BMD Z-scores in subjects randomized to placebo were −0.28 for lumbar spine and −0.26 for total body. In subjects receiving VIREAD for 72 weeks, the mean change in BMD Z-score was −0.05 for lumbar spine and −0.15 for total body compared to +0.07 and +0.06, respectively, in subjects receiving placebo. As observed in pediatric studies of HIV-infected patients, skeletal growth (height) appeared to be unaffected [See Warnings and Precautions (5.6)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of VIREAD. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders
allergic reaction, including angioedemaMetabolism and Nutrition Disorders
lactic acidosis, hypokalemia, hypophosphatemiaRespiratory, Thoracic, and Mediastinal Disorders
dyspneaGastrointestinal Disorders
pancreatitis, increased amylase, abdominal painHepatobiliary Disorders
hepatic steatosis, hepatitis, increased liver enzymes (most commonly AST, ALT gamma GT)Skin and Subcutaneous Tissue Disorders
rashMusculoskeletal and Connective Tissue Disorders
rhabdomyolysis, osteomalacia (manifested as bone pain and which may contribute to fractures), muscular weakness, myopathyRenal and Urinary Disorders
acute renal failure, renal failure, acute tubular necrosis, Fanconi syndrome, proximal renal tubulopathy, interstitial nephritis (including acute cases), nephrogenic diabetes insipidus, renal insufficiency, increased creatinine, proteinuria, polyuriaGeneral Disorders and Administration Site Conditions
astheniaThe following adverse reactions, listed under the body system headings above, may occur as a consequence of proximal renal tubulopathy: rhabdomyolysis, osteomalacia, hypokalemia, muscular weakness, myopathy, hypophosphatemia.
-
7 DRUG INTERACTIONS
This section describes clinically relevant drug interactions with VIREAD. Drug interactions trials are described elsewhere in the labeling [See Clinical Pharmacology (12.3)].
7.1 Didanosine
Coadministration of VIREAD and didanosine should be undertaken with caution and patients receiving this combination should be monitored closely for didanosine-associated adverse reactions. Didanosine should be discontinued in patients who develop didanosine-associated adverse reactions.
When administered with VIREAD, Cmax and AUC of didanosine increased significantly [See Clinical Pharmacology (12.3)]. The mechanism of this interaction is unknown. Higher didanosine concentrations could potentiate didanosine-associated adverse reactions, including pancreatitis and neuropathy. Suppression of CD4+ cell counts has been observed in patients receiving VIREAD with didanosine 400 mg daily.
In patients weighing greater than 60 kg, the didanosine dose should be reduced to 250 mg once daily when it is coadministered with VIREAD. In patients weighing less than 60 kg, the didanosine dose should be reduced to 200 mg once daily when it is coadministered with VIREAD. When coadministered, VIREAD and didanosine EC may be taken under fasted conditions or with a light meal (less than 400 kcal, 20% fat). For additional information on coadministration of VIREAD and didanosine, please refer to the full prescribing information for didanosine.
7.2 HIV-1 Protease Inhibitors
VIREAD decreases the AUC and Cmin of atazanavir [See Clinical Pharmacology (12.3)]. When coadministered with VIREAD, it is recommended that atazanavir 300 mg is given with ritonavir 100 mg. VIREAD should not be coadministered with atazanavir without ritonavir.
Lopinavir/ritonavir, atazanavir coadministered with ritonavir, and darunavir coadministered with ritonavir have been shown to increase tenofovir concentrations [See Clinical Pharmacology (12.3)]. Tenofovir disoproxil fumarate is a substrate of P-glycoprotein (Pgp) and breast cancer resistance protein (BCRP) transporters. When tenofovir disoproxil fumarate is coadministered with an inhibitor of these transporters, an increase in absorption may be observed. Patients receiving VIREAD concomitantly with lopinavir/ritonavir, ritonavir-boosted atazanavir, or ritonavir-boosted darunavir should be monitored for VIREAD-associated adverse reactions. VIREAD should be discontinued in patients who develop VIREAD-associated adverse reactions.
7.3 Drugs Affecting Renal Function
Since tenofovir is primarily eliminated by the kidneys [See Clinical Pharmacology (12.3)], coadministration of VIREAD with drugs that reduce renal function or compete for active tubular secretion may increase serum concentrations of tenofovir and/or increase the concentrations of other renally eliminated drugs. Some examples include, but are not limited to, cidofovir, acyclovir, valacyclovir, ganciclovir, valganciclovir, aminoglycosides (e.g., gentamicin), and high-dose or multiple NSAIDs [See Warnings and Precautions (5.3)].
In the treatment of chronic hepatitis B, VIREAD should not be administered in combination with HEPSERA (adefovir dipivoxil).
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, VIREAD should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
Nursing Mothers: The Centers for Disease Control and Prevention recommend that HIV-1-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV-1. Samples of breast milk obtained from five HIV-1 infected mothers in the first post-partum week show that tenofovir is secreted in human milk. The impact of this exposure in breastfed infants is unknown. Because of both the potential for HIV-1 transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving VIREAD.
8.4 Pediatric Use
Pediatric Patients 2 Years of Age and Older with HIV-1 infection
The safety of VIREAD in pediatric patients aged 2 to less than 18 years is supported by data from two randomized trials in which VIREAD was administered to HIV-1 infected treatment-experienced subjects. In addition, the pharmacokinetic profile of tenofovir in patients 2 to less than 18 years of age at the recommended doses was similar to that found to be safe and effective in adult clinical trials [See Clinical Pharmacology (12.3)].
In Study 352, 92 treatment-experienced subjects 2 to less than 12 years of age with stable, virologic suppression on stavudine- or zidovudine-containing regimen were randomized to either replace stavudine or zidovudine with VIREAD (N = 44) or continue their original regimen (N = 48) for 48 weeks. Five additional subjects over the age of 12 were enrolled and randomized (VIREAD N=4, original regimen N=1) but are not included in the efficacy analysis. After 48 weeks, all eligible subjects were allowed to continue in the study receiving open-label VIREAD. At Week 48, 89% of subjects in the VIREAD treatment group and 90% of subjects in the stavudine or zidovudine treatment group had HIV-1 RNA concentrations less than 400 copies/mL. During the 48 week randomized phase of the study, 1 subject in the VIREAD group discontinued the study prematurely because of virologic failure/lack of efficacy and 3 subjects (2 subjects in the VIREAD group and 1 subject in the stavudine or zidovudine group) discontinued for other reasons.
In Study 321, 87 treatment-experienced subjects 12 to less than 18 years of age were treated with VIREAD (N=45) or placebo (N=42) in combination with an optimized background regimen (OBR) for 48 weeks. The mean baseline CD4 cell count was 374 cells/mm3 and the mean baseline plasma HIV-1 RNA was 4.6 log10 copies/mL. At baseline, 90% of subjects harbored NRTI resistance-associated substitutions in their HIV-1 isolates. Overall, the trial failed to show a difference in virologic response between the VIREAD and placebo treatment groups. Subgroup analyses suggest the lack of difference in virologic response may be attributable to imbalances between treatment arms in baseline viral susceptibility to VIREAD and OBR.
Although changes in HIV-1 RNA in these highly treatment-experienced subjects were less than anticipated, the comparability of the pharmacokinetic and safety data to that observed in adults supports the use of VIREAD in pediatric patients 12 years of age and older who weigh greater than or equal to 35 kg and whose HIV-1 isolate is expected to be sensitive to VIREAD. [See Warnings and Precautions (5.6), Adverse Reactions (6.1), and Clinical Pharmacology (12.3)].
Safety and effectiveness of VIREAD in pediatric patients younger than 2 years of age with HIV-1 infection have not been established.
Pediatric Patients 12 Years of Age and Older with Chronic Hepatitis B
In Study 115, 106 HBeAg negative (9%) and positive (91%) subjects aged 12 to less than 18 years with chronic HBV infection were randomized to receive blinded treatment with VIREAD 300 mg (N = 52) or placebo (N = 54) for 72 weeks. At study entry, the mean HBV DNA was 8.1 log10 copies/mL and mean ALT was 101 U/L. Of 52 subjects treated with VIREAD, 20 subjects were nucleos(t)ide-naïve and 32 subjects were nucleos(t)ide-experienced. Thirty-one of the 32 nucleos(t)ide-experienced subjects had prior lamivudine experience. At Week 72, 88% (46/52) of subjects in the VIREAD group and 0% (0/54) of subjects in the placebo group had HBV DNA <400 copies/mL (69 IU/mL). Among subjects with abnormal ALT at baseline, 74% (26/35) of subjects receiving VIREAD had normalized ALT at Week 72 compared to 31% (13/42) in the placebo group. One VIREAD-treated subject experienced sustained HBsAg-loss and seroconversion to anti-HBs during the first 72 weeks of study participation.
Safety and effectiveness of VIREAD in pediatric patients younger than 12 years of age or less than 35 kg with chronic hepatitis B have not been established.
8.5 Geriatric Use
Clinical trials of VIREAD did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for the elderly patient should be cautious, keeping in mind the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Patients with Impaired Renal Function
It is recommended that the dosing interval for VIREAD be modified in patients with estimated creatinine clearance below 50 mL/min or in patients with ESRD who require dialysis [See Dosage and Administration (2.3), Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Limited clinical experience at doses higher than the therapeutic dose of VIREAD 300 mg is available. In Study 901, 600 mg tenofovir disoproxil fumarate was administered to 8 subjects orally for 28 days. No severe adverse reactions were reported. The effects of higher doses are not known.
If overdose occurs the patient must be monitored for evidence of toxicity, and standard supportive treatment applied as necessary.
Tenofovir is efficiently removed by hemodialysis with an extraction coefficient of approximately 54%. Following a single 300 mg dose of VIREAD, a four-hour hemodialysis session removed approximately 10% of the administered tenofovir dose.
-
11 DESCRIPTION
VIREAD is the brand name for tenofovir disoproxil fumarate (a prodrug of tenofovir) which is a fumaric acid salt of bis-isopropoxycarbonyloxymethyl ester derivative of tenofovir. In vivo tenofovir disoproxil fumarate is converted to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5'-monophosphate. Tenofovir exhibits activity against HIV-1 reverse transcriptase.
The chemical name of tenofovir disoproxil fumarate is 9-[(R)-2-[[bis[[(isopropoxycarbonyl)oxy]methoxy]phosphinyl]methoxy]propyl]adenine fumarate (1:1). It has a molecular formula of C19H30N5O10P • C4H4O4 and a molecular weight of 635.52. It has the following structural formula:
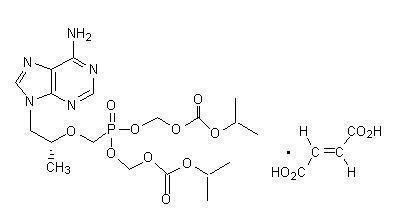
Tenofovir disoproxil fumarate is a white to off-white crystalline powder with a solubility of 13.4 mg/mL in distilled water at 25 °C. It has an octanol/phosphate buffer (pH 6.5) partition coefficient (log p) of 1.25 at 25 °C.
VIREAD is available as tablets or as an oral powder.
VIREAD tablets are for oral administration in strengths of 150, 200, 250, and 300 mg of tenofovir disoproxil fumarate, which are equivalent to 123, 163, 204 and 245 mg of tenofovir disoproxil, respectively. Each tablet contains the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and pregelatinized starch. The 300 mg tablets are coated with Opadry II Y-30-10671-A, which contains FD&C blue #2 aluminum lake, hypromellose 2910, lactose monohydrate, titanium dioxide, and triacetin. The 150, 200, and 250 mg tablets are coated with Opadry II 32K-18425, which contains hypromellose 2910, lactose monohydrate, titanium dioxide, and triacetin.
VIREAD oral powder is available for oral administration as white, taste-masked, coated granules containing 40 mg of tenofovir disoproxil fumarate per gram of oral powder, which is equivalent to 33 mg of tenofovir disoproxil. The oral powder contains the following inactive ingredients: mannitol, hydroxypropyl cellulose, ethylcellulose, and silicon dioxide.
In this insert, all dosages are expressed in terms of tenofovir disoproxil fumarate except where otherwise noted.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tenofovir disoproxil fumarate is an antiviral drug [See Microbiology (12.4)].
12.3 Pharmacokinetics
The pharmacokinetics of tenofovir disoproxil fumarate have been evaluated in healthy volunteers and HIV-1 infected individuals. Tenofovir pharmacokinetics are similar between these populations.
Absorption
VIREAD is a water soluble diester prodrug of the active ingredient tenofovir. The oral bioavailability of tenofovir from VIREAD in fasted subjects is approximately 25%. Following oral administration of a single dose of VIREAD 300 mg to HIV-1 infected subjects in the fasted state, maximum serum concentrations (Cmax) are achieved in 1.0 ± 0.4 hrs. Cmax and AUC values are 0.30 ± 0.09 µg/mL and 2.29 ± 0.69 µg∙hr/mL, respectively.
The pharmacokinetics of tenofovir are dose proportional over a VIREAD dose range of 75 to 600 mg and are not affected by repeated dosing.
In a single-dose bioequivalence study conducted under non-fasted conditions (dose administered with 4 oz. applesauce) in healthy adult volunteers, the mean Cmax of tenofovir was 26% lower for the oral powder relative to the tablet formulation. Mean AUC of tenofovir was similar between the oral powder and tablet formulations.
Distribution
In vitro binding of tenofovir to human plasma or serum proteins is less than 0.7 and 7.2%, respectively, over the tenofovir concentration range 0.01 to 25 µg/mL. The volume of distribution at steady-state is 1.3 ± 0.6 L/kg and 1.2 ± 0.4 L/kg, following intravenous administration of tenofovir 1.0 mg/kg and 3.0 mg/kg.
Metabolism and Elimination
In vitro studies indicate that neither tenofovir disoproxil nor tenofovir are substrates of CYP enzymes.
Following IV administration of tenofovir, approximately 70–80% of the dose is recovered in the urine as unchanged tenofovir within 72 hours of dosing. Following single dose, oral administration of VIREAD, the terminal elimination half-life of tenofovir is approximately 17 hours. After multiple oral doses of VIREAD 300 mg once daily (under fed conditions), 32 ± 10% of the administered dose is recovered in urine over 24 hours.
Tenofovir is eliminated by a combination of glomerular filtration and active tubular secretion. There may be competition for elimination with other compounds that are also renally eliminated.
Effects of Food on Oral Absorption
Administration of VIREAD 300 mg tablets following a high-fat meal (~700 to 1000 kcal containing 40 to 50% fat) increases the oral bioavailability, with an increase in tenofovir AUC0–∞ of approximately 40% and an increase in Cmax of approximately 14%. However, administration of VIREAD with a light meal did not have a significant effect on the pharmacokinetics of tenofovir when compared to fasted administration of the drug. Food delays the time to tenofovir Cmax by approximately 1 hour. Cmax and AUC of tenofovir are 0.33 ± 0.12 µg/mL and 3.32 ± 1.37 µg∙hr/mL following multiple doses of VIREAD 300 mg once daily in the fed state, when meal content was not controlled.
Special Populations
Race: There were insufficient numbers from racial and ethnic groups other than Caucasian to adequately determine potential pharmacokinetic differences among these populations.
Pediatric Patients 2 Years of Age and Older: Steady-state pharmacokinetics of tenofovir were evaluated in 31 HIV-1 infected pediatric subjects 2 to less than 18 years (Table 11). Tenofovir exposure achieved in these pediatric subjects receiving oral once daily doses of VIREAD 300 mg (tablet) or 8 mg/kg of body weight (powder) up to a maximum dose of 300 mg was similar to exposures achieved in adults receiving once-daily doses of VIREAD 300 mg.
Table 11 Mean (± SD) Tenofovir Pharmacokinetic Parameters by Age Groups for HIV-1-infected Pediatric Patients Dose and Formulation 300 mg Tablet 8 mg/kg Oral Powder 12 to <18 Years (N=8) 2 to <12 Years (N=23) Cmax (µg/mL) 0.38 ± 0.13 0.24 ± 0.13 AUCtau (µg∙hr/mL) 3.39 ± 1.22 2.59 ± 1.06 Tenofovir exposures in 52 HBV-infected pediatric subjects (12 to less than 18 years of age) receiving oral once-daily doses of VIREAD 300 mg tablet were comparable to exposures achieved in HIV-1-infected adults and adolescents receiving once-daily doses of 300 mg.
Geriatric Patients: Pharmacokinetic trials have not been performed in the elderly (65 years and older).
Patients with Impaired Renal Function: The pharmacokinetics of tenofovir are altered in subjects with renal impairment [See Warnings and Precautions (5.3)]. In subjects with creatinine clearance below 50 mL/min or with end-stage renal disease (ESRD) requiring dialysis, Cmax, and AUC0–∞ of tenofovir were increased (Table 12). It is recommended that the dosing interval for VIREAD be modified in patients with estimated creatinine clearance below 50 mL/min or in patients with ESRD who require dialysis [See Dosage and Administration (2.3)].
Table 12 Pharmacokinetic Parameters (Mean ± SD) of Tenofovir* in Subjects with Varying Degrees of Renal Function Baseline Creatinine Clearance (mL/min) >80
(N=3)50–80
(N=10)30–49
(N=8)12–29
(N=11)- *
- 300 mg, single dose of VIREAD
Cmax (µg/mL) 0.34 ± 0.03 0.33 ± 0.06 0.37 ± 0.16 0.60 ± 0.19 AUC0–∞ (µg∙hr/mL) 2.18 ± 0.26 3.06 ± 0.93 6.01 ± 2.50 15.98 ± 7.22 CL/F (mL/min) 1043.7 ± 115.4 807.7 ± 279.2 444.4 ± 209.8 177.0 ± 97.1 CLrenal (mL/min) 243.5 ± 33.3 168.6 ± 27.5 100.6 ± 27.5 43.0 ± 31.2 Tenofovir is efficiently removed by hemodialysis with an extraction coefficient of approximately 54%. Following a single 300 mg dose of VIREAD, a four-hour hemodialysis session removed approximately 10% of the administered tenofovir dose.
Patients with Hepatic Impairment: The pharmacokinetics of tenofovir following a 300 mg single dose of VIREAD have been studied in non-HIV infected subjects with moderate to severe hepatic impairment. There were no substantial alterations in tenofovir pharmacokinetics in subjects with hepatic impairment compared with unimpaired subjects. No change in VIREAD dosing is required in patients with hepatic impairment.
Assessment of Drug Interactions
At concentrations substantially higher (~300-fold) than those observed in vivo, tenofovir did not inhibit in vitro drug metabolism mediated by any of the following human CYP isoforms: CYP3A4, CYP2D6, CYP2C9, or CYP2E1. However, a small (6%) but statistically significant reduction in metabolism of CYP1A substrate was observed. Based on the results of in vitro experiments and the known elimination pathway of tenofovir, the potential for CYP mediated interactions involving tenofovir with other medicinal products is low.
VIREAD has been evaluated in healthy volunteers in combination with other antiretroviral and potential concomitant drugs. Tables 13 and 14 summarize pharmacokinetic effects of coadministered drug on tenofovir pharmacokinetics and effects of VIREAD on the pharmacokinetics of coadministered drug. Coadministration of VIREAD with didanosine results in changes in the pharmacokinetics of didanosine that may be of clinical significance. Concomitant dosing of VIREAD with didanosine significantly increases the Cmax and AUC of didanosine. When didanosine 250 mg enteric-coated capsules were administered with VIREAD, systemic exposures of didanosine were similar to those seen with the 400 mg enteric-coated capsules alone under fasted conditions (Table 14). The mechanism of this interaction is unknown.
No clinically significant drug interactions have been observed between VIREAD and efavirenz, methadone, nelfinavir, oral contraceptives, or ribavirin.
Table 13 Drug Interactions: Changes in Pharmacokinetic Parameters for Tenofovir* in the Presence of the Coadministered Drug Coadministered Drug Dose of Coadministered Drug (mg) N % Change of Tenofovir Pharmacokinetic Parameters†
(90% CI)Cmax AUC Cmin Abacavir 300 once 8 

NC Atazanavir‡ 400 once daily
× 14 days33 ↑ 14
(↑ 8 to ↑ 20)↑ 24
(↑ 21 to ↑ 28)↑ 22
(↑ 15 to ↑ 30)Atazanavir/ Ritonavir‡ 300/100 once daily 12 ↑ 34
(↑ 20 to ↑ 51)↑ 37
(↑ 30 to ↑ 45)↑ 29
(↑ 21 to ↑ 36)Darunavir/ Ritonavir§ 300/100 twice daily 12 ↑ 24
(↑ 8 to ↑ 42)↑ 22
(↑ 10 to ↑ 35)↑ 37
(↑ 19 to ↑ 57)Didanosine¶ 250 or 400 once daily × 7 days 14 


Emtricitabine 200 once daily
× 7 days17 


Entecavir 1 mg once daily ×
10 days28 


Indinavir 800 three times daily × 7 days 13 ↑ 14
(↓ 3 to ↑ 33)

Lamivudine 150 twice daily
× 7 days15 


Lopinavir/Ritonavir 400/100 twice daily × 14 days 24 
↑ 32
(↑ 25 to ↑ 38)↑ 51
(↑ 37 to ↑ 66)Saquinavir/Ritonavir 1000/100 twice daily × 14 days 35 

↑ 23
(↑ 16 to ↑ 30)Tacrolimus 0.05 mg/kg twice daily × 7 days 21 ↑ 13
(↑ 1 to ↑ 27)

Tipranavir/ Ritonavir# 500/100 twice daily 22 ↓ 23
(↓ 32 to ↓ 13)↓ 2
(↓ 9 to ↑ 5)↑ 7
(↓ 2 to ↑ 17)750/200 twice daily (23 doses) 20 ↓ 38
(↓ 46 to ↓ 29)↑ 2
(↓ 6 to ↑ 10)↑ 14
(↑ 1 to ↑ 27)Table 14 Drug Interactions: Changes in Pharmacokinetic Parameters for Coadministered Drug in the Presence of VIREAD Coadministered Drug Dose of Coadministered Drug (mg) N % Change of Coadministered Drug Pharmacokinetic Parameters*
(90% CI)Cmax AUC Cmin - *
-
Increase = ↑; Decrease = ↓; No Effect =
 ; NA = Not Applicable
; NA = Not Applicable - †
- Reyataz Prescribing Information
- ‡
- In HIV-infected subjects, addition of tenofovir DF to atazanavir 300 mg plus ritonavir 100 mg, resulted in AUC and Cmin values of atazanavir that were 2.3- and 4-fold higher than the respective values observed for atazanavir 400 mg when given alone.
- §
- Prezista Prescribing Information
- ¶
- Videx EC Prescribing Information. Subjects received didanosine enteric-coated capsules.
- #
- 373 kcal, 8.2 g fat
- Þ
- Compared with didanosine (enteric-coated) 400 mg administered alone under fasting conditions.
- ß
- Increases in AUC and Cmin are not expected to be clinically relevant; hence no dose adjustments are required when tenofovir DF and ritonavir-boosted saquinavir are coadministered.
- à
- Aptivus Prescribing Information
Abacavir 300 once 8 ↑ 12
(↓ 1 to ↑ 26)
NA Atazanavir† 400 once daily
× 14 days34 ↓ 21
(↓ 27 to ↓ 14)↓ 25
(↓ 30 to ↓ 19)↓ 40
(↓ 48 to ↓ 32)Atazanavir† Atazanavir/ Ritonavir
300/100 once daily
× 42 days10 ↓ 28
(↓ 50 to ↑ 5)↓ 25‡
(↓ 42 to ↓ 3)↓ 23‡
(↓ 46 to ↑ 10)Darunavir§ Darunavir/Ritonavir 300/100 mg once daily 12 ↑ 16
(↓ 6 to ↑ 42)↑ 21
(↓ 5 to ↑ 54)↑ 24
(↓ 10 to ↑ 69)Didanosine¶ 250 once, simultaneously with VIREAD and a light meal# 33 ↓ 20Þ
(↓ 32 to ↓ 7) Þ
ÞNA Emtricitabine 200 once daily
× 7 days17 

↑ 20
(↑ 12 to ↑ 29)Entecavir 1 mg once daily
× 10 days28 
↑ 13
(↑ 11 to ↑ 15)
Indinavir 800 three times daily × 7 days 12 ↓ 11
(↓ 30 to ↑ 12)

Lamivudine 150 twice daily
× 7 days15 ↓ 24
(↓ 34 to ↓ 12)

Lopinavir Lopinavir/Ritonavir 400/100 twice daily × 14 days 24 


Ritonavir 


Saquinavir Saquinavir/Ritonavir 1000/100 twice daily × 14 days 32 ↑ 22
(↑ 6 to ↑ 41)↑ 29ß
(↑ 12 to ↑ 48)↑ 47ß
(↑ 23 to ↑ 76)Ritonavir 

↑ 23
(↑ 3 to ↑ 46)Tacrolimus 0.05 mg/kg twice
daily × 7 days21 


Tipranavirà Tipranavir/Ritonavir 500/100 twice daily 22 ↓ 17
(↓ 26 to ↓ 6)↓ 18
(↓ 25 to ↓ 9)↓ 21
(↓ 30 to ↓ 10)Tipranavir/Ritonavir 750/200 twice daily (23 doses) 20 ↓ 11
(↓ 16 to ↓ 4)↓ 9
(↓ 15 to ↓ 3)↓ 12
(↓ 22 to 0)12.4 Microbiology
Mechanism of Action
Tenofovir disoproxil fumarate is an acyclic nucleoside phosphonate diester analog of adenosine monophosphate. Tenofovir disoproxil fumarate requires initial diester hydrolysis for conversion to tenofovir and subsequent phosphorylations by cellular enzymes to form tenofovir diphosphate, an obligate chain terminator. Tenofovir diphosphate inhibits the activity of HIV-1 reverse transcriptase and HBV reverse transcriptase by competing with the natural substrate deoxyadenosine 5'-triphosphate and, after incorporation into DNA, by DNA chain termination. Tenofovir diphosphate is a weak inhibitor of mammalian DNA polymerases α, β, and mitochondrial DNA polymerase γ.
Activity against HIV
Antiviral Activity
The antiviral activity of tenofovir against laboratory and clinical isolates of HIV-1 was assessed in lymphoblastoid cell lines, primary monocyte/macrophage cells and peripheral blood lymphocytes. The EC50 (50% effective concentration) values for tenofovir were in the range of 0.04 µM to 8.5 µM. In drug combination studies, tenofovir was not antagonistic with nucleoside reverse transcriptase inhibitors (abacavir, didanosine, lamivudine, stavudine, zalcitabine, zidovudine), non-nucleoside reverse transcriptase inhibitors (delavirdine, efavirenz, nevirapine), and protease inhibitors (amprenavir, indinavir, nelfinavir, ritonavir, saquinavir). Tenofovir displayed antiviral activity in cell culture against HIV-1 clades A, B, C, D, E, F, G, and O (EC50 values ranged from 0.5 µM to 2.2 µM) and strain specific activity against HIV-2 (EC50 values ranged from 1.6 µM to 5.5 µM).
Resistance
HIV-1 isolates with reduced susceptibility to tenofovir have been selected in cell culture. These viruses expressed a K65R substitution in reverse transcriptase and showed a 2- to 4- fold reduction in susceptibility to tenofovir.
In Study 903 of treatment-naïve subjects (VIREAD + lamivudine + efavirenz versus stavudine + lamivudine + efavirenz) [See Clinical Studies (14.1)], genotypic analyses of isolates from subjects with virologic failure through Week 144 showed development of efavirenz and lamivudine resistance-associated substitutions to occur most frequently and with no difference between the treatment arms. The K65R substitution occurred in 8/47 (17%) analyzed patient isolates on the VIREAD arm and in 2/49 (4%) analyzed patient isolates on the stavudine arm. Of the 8 subjects whose virus developed K65R in the VIREAD arm through 144 weeks, 7 of these occurred in the first 48 weeks of treatment and one at Week 96. Other substitutions resulting in resistance to VIREAD were not identified in this trial.
In Study 934 of treatment-naïve subjects (VIREAD + EMTRIVA + efavirenz versus zidovudine (AZT)/lamivudine (3TC) + efavirenz) [See Clinical Studies (14.1)], genotypic analysis performed on HIV-1 isolates from all confirmed virologic failure subjects with greater than 400 copies/mL of HIV-1 RNA at Week 144 or early discontinuation showed development of efavirenz resistance-associated substitutions occurred most frequently and was similar between the two treatment arms. The M184V substitution, associated with resistance to EMTRIVA and lamivudine, was observed in 2/19 analyzed subject isolates in the VIREAD + EMTRIVA group and in 10/29 analyzed subject isolates in the zidovudine/lamivudine group. Through 144 weeks of Study 934, no subjects have developed a detectable K65R substitution in their HIV-1 as analyzed through standard genotypic analysis.
Cross-Resistance
Cross-resistance among certain reverse transcriptase inhibitors has been recognized. The K65R substitution selected by tenofovir is also selected in some HIV-1 infected subjects treated with abacavir, didanosine, or zalcitabine. HIV-1 isolates with this substitution also show reduced susceptibility to emtricitabine and lamivudine. Therefore, cross-resistance among these drugs may occur in patients whose virus harbors the K65R substitution. HIV-1 isolates from subjects (N=20) whose HIV-1 expressed a mean of 3 zidovudine-associated reverse transcriptase substitutions (M41L, D67N, K70R, L210W, T215Y/F, or K219Q/E/N), showed a 3.1-fold decrease in the susceptibility to tenofovir.
In Studies 902 and 907 conducted in treatment-experienced subjects (VIREAD + Standard Background Therapy (SBT) compared to Placebo + SBT) [See Clinical Studies (14.1)], 14/304 (5%) of the VIREAD-treated subjects with virologic failure through Week 96 had greater than 1.4-fold (median 2.7-fold) reduced susceptibility to tenofovir. Genotypic analysis of the baseline and failure isolates showed the development of the K65R substitution in the HIV-1 reverse transcriptase gene.
The virologic response to VIREAD therapy has been evaluated with respect to baseline viral genotype (N=222) in treatment-experienced subjects participating in Studies 902 and 907. In these clinical trials, 94% of the participants evaluated had baseline HIV-1 isolates expressing at least one NRTI substitution. Virologic responses for subjects in the genotype substudy were similar to the overall trial results.
Several exploratory analyses were conducted to evaluate the effect of specific substitutions and substitutional patterns on virologic outcome. Because of the large number of potential comparisons, statistical testing was not conducted. Varying degrees of cross-resistance of VIREAD to pre-existing zidovudine resistance-associated substitutions (M41L, D67N, K70R, L210W, T215Y/F, or K219Q/E/N) were observed and appeared to depend on the type and number of specific substitutions. VIREAD-treated subjects whose HIV-1 expressed 3 or more zidovudine resistance-associated substitutions that included either the M41L or L210W reverse transcriptase substitution showed reduced responses to VIREAD therapy; however, these responses were still improved compared with placebo. The presence of the D67N, K70R, T215Y/F, or K219Q/E/N substitution did not appear to affect responses to VIREAD therapy. Subjects whose virus expressed an L74V substitution without zidovudine resistance associated substitutions (N=8) had reduced response to VIREAD. Limited data are available for subjects whose virus expressed a Y115F substitution (N=3), Q151M substitution (N=2), or T69 insertion (N=4), all of whom had a reduced response.
In the protocol defined analyses, virologic response to VIREAD was not reduced in subjects with HIV-1 that expressed the abacavir/emtricitabine/lamivudine resistance-associated M184V substitution. HIV-1 RNA responses among these subjects were durable through Week 48.
Studies 902 and 907 Phenotypic Analyses
Phenotypic analysis of baseline HIV-1 from treatment-experienced subjects (N=100) demonstrated a correlation between baseline susceptibility to VIREAD and response to VIREAD therapy. Table 15 summarizes the HIV-1 RNA response by baseline VIREAD susceptibility.
Activity against HBV
Antiviral Activity
The antiviral activity of tenofovir against HBV was assessed in the HepG2 2.2.15 cell line. The EC50 values for tenofovir ranged from 0.14 to 1.5 µM, with CC50 (50% cytotoxicity concentration) values greater than 100 µM. In cell culture combination antiviral activity studies of tenofovir with the nucleoside HBV reverse transcriptase inhibitors entecavir, lamivudine, and telbivudine, and with the nucleoside HIV-1 reverse transcriptase inhibitor emtricitabine, no antagonistic activity was observed.
Resistance
Cumulative VIREAD genotypic resistance has been evaluated annually for up to 384 weeks in Studies 0102, 0103, 0106, 0108, and 0121 with the paired HBV reverse transcriptase amino acid sequences of the pre-treatment and on-treatment isolates from subjects who received at least 24 weeks of VIREAD monotherapy and remained viremic with HBV DNA greater than or equal to 400 copies/mL (69 IU/mL) at the end of each study year (or at discontinuation of VIREAD monotherapy) using an as-treated analysis. In the nucleotide-naïve population from Studies 0102 and 0103, HBeAg-positive subjects had a higher baseline viral load than HBeAg-negative subjects and a significantly higher proportion of the subjects remained viremic at their last time point on VIREAD monotherapy (15% versus 5%, respectively).
HBV isolates from these subjects who remained viremic showed treatment-emergent substitutions (Table 16); however, no specific substitutions occurred at a sufficient frequency to be associated with resistance to VIREAD (genotypic and phenotypic analyses).
Table 16 Amino Acid Substitutions in Viremic Subjects Across HBV Trials of VIREAD Compensated Liver Disease Decompensated Liver Disease (N=39)* Nucleotide-Naïve (N=417)† HEPSERA-Experienced (N=247)‡ Lamivudine- Resistant (N=136)§ - *
- Subjects with decompensated liver disease from Study 0108 (N=39) receiving up to 48 weeks of treatment with VIREAD.
- †
- Nucleotide-naïve subjects from Studies 0102 (N=246) and 0103 (N=171) receiving up to 384 weeks of treatment with VIREAD.
- ‡
- HEPSERA-experienced subjects from Studies 0102/0103 (N=195) and 0106 (N=52) receiving up to 336 weeks of treatment with VIREAD after switching to VIREAD from HEPSERA. Study 0106, a randomized, double-blind, 168-week Phase 2 trial, has been completed.
- §
- Lamivudine-resistant subjects from Study 0121 (N=136) receiving up to 96 weeks of treatment with VIREAD after switching to VIREAD from lamivudine.
- ¶
- Denominator includes those subjects who were viremic at last time point on VIREAD monotherapy and had evaluable paired genotypic data.
- #
- Of the 18 subjects with treatment-emergent amino acid substitutions during Studies 0102 and 0103, 5 subjects had substitutions at conserved sites and 13 subjects had substitutions only at polymorphic sites, and 8 subjects had only transient substitutions that were not detected at the last time point on VIREAD.
- Þ
- Of the 11 HEPSERA-experienced subjects with treatment-emergent amino acid substitutions, 2 subjects had substitutions at conserved sites and 9 had substitutions only at polymorphic sites.
- ß
- Of the 6 lamivudine-resistant subjects with treatment-emergent substitutions during Study 0121, 3 subjects had substitutions at conserved sites and 3 had substitutions only at polymorphic sites.
Viremic at Last Time Point on VIREAD 38/417 (9%) 37/247 (15%) 9/136 (7%) 7/39 (18%) Treatment-Emergent Amino Acid Substitutions¶ 18#/32 (56%) 11Þ/31 (35%) 6ß/8 (75%) 3/5 (60%) Cross-Resistance
Cross-resistance has been observed between HBV nucleoside/nucleotide analogue reverse transcriptase inhibitors.
In cell based assays, HBV strains expressing the rtV173L, rtL180M, and rtM204I/V substitutions associated with resistance to lamivudine and telbivudine showed a susceptibility to tenofovir ranging from 0.7- to 3.4-fold that of wild type virus. The rtL180M and rtM204I/V double substitutions conferred 3.4-fold reduced susceptibility to tenofovir.
HBV strains expressing the rtL180M, rtT184G, rtS202G/I, rtM204V, and rtM250V substitutions associated with resistance to entecavir showed a susceptibility to tenofovir ranging from 0.6- to 6.9-fold that of wild type virus.
HBV strains expressing the adefovir resistance-associated substitutions rtA181V and/or rtN236T showed reductions in susceptibility to tenofovir ranging from 2.9- to 10-fold that of wild type virus. Strains containing the rtA181T substitution showed changes in susceptibility to tenofovir ranging from 0.9- to 1.5-fold that of wild type virus.
One hundred fifty-two subjects initiating VIREAD therapy in Studies 0102, 0103, 0106, 0108, and 0121 harbored HBV with known resistance substitutions to HBV nucleos(t)ide analogue reverse transcriptase inhibitors: 14 with adefovir resistance-associated substitutions (rtA181S/T/V and/or rtN236T), 135 with lamivudine resistance-associated substitutions (rtM204I/V), and 3 with both adefovir and lamivudine resistance-associated substitutions. Following up to 384 weeks of VIREAD treatment, 10 of the 14 subjects with adefovir-resistant HBV, 124 of the 135 subjects with lamivudine-resistant HBV, and 2 of the 3 subjects with both adefovir- and lamivudine-resistant HBV achieved and maintained virologic suppression (HBV DNA less than 400 copies/mL [69 IU/mL]). Three of the 5 subjects whose virus harbored both the rtA181T/V and rtN236T substitutions remained viremic.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term oral carcinogenicity studies of tenofovir disoproxil fumarate in mice and rats were carried out at exposures up to approximately 16 times (mice) and 5 times (rats) those observed in humans at the therapeutic dose for HIV-1 infection. At the high dose in female mice, liver adenomas were increased at exposures 16 times that in humans. In rats, the study was negative for carcinogenic findings at exposures up to 5 times that observed in humans at the therapeutic dose.
Mutagenesis
Tenofovir disoproxil fumarate was mutagenic in the in vitro mouse lymphoma assay and negative in an in vitro bacterial mutagenicity test (Ames test). In an in vivo mouse micronucleus assay, tenofovir disoproxil fumarate was negative when administered to male mice.
Impairment of Fertility
There were no effects on fertility, mating performance or early embryonic development when tenofovir disoproxil fumarate was administered to male rats at a dose equivalent to 10 times the human dose based on body surface area comparisons for 28 days prior to mating and to female rats for 15 days prior to mating through day seven of gestation. There was, however, an alteration of the estrous cycle in female rats.
13.2 Animal Toxicology and/or Pharmacology
Tenofovir and tenofovir disoproxil fumarate administered in toxicology studies to rats, dogs, and monkeys at exposures (based on AUCs) greater than or equal to 6 fold those observed in humans caused bone toxicity. In monkeys the bone toxicity was diagnosed as osteomalacia. Osteomalacia observed in monkeys appeared to be reversible upon dose reduction or discontinuation of tenofovir. In rats and dogs, the bone toxicity manifested as reduced bone mineral density. The mechanism(s) underlying bone toxicity is unknown.
Evidence of renal toxicity was noted in 4 animal species. Increases in serum creatinine, BUN, glycosuria, proteinuria, phosphaturia, and/or calciuria and decreases in serum phosphate were observed to varying degrees in these animals. These toxicities were noted at exposures (based on AUCs) 2–20 times higher than those observed in humans. The relationship of the renal abnormalities, particularly the phosphaturia, to the bone toxicity is not known.
-
14 CLINICAL STUDIES
14.1 Clinical Efficacy in Adults with HIV-1 Infection
Treatment-Naïve Adult Patients
Study 903
Data through 144 weeks are reported for Study 903, a double-blind, active-controlled multicenter trial comparing VIREAD (300 mg once daily) administered in combination with lamivudine and efavirenz versus stavudine (d4T), lamivudine, and efavirenz in 600 antiretroviral-naïve subjects. Subjects had a mean age of 36 years (range 18–64), 74% were male, 64% were Caucasian and 20% were Black. The mean baseline CD4+ cell count was 279 cells/mm3 (range 3–956) and median baseline plasma HIV-1 RNA was 77,600 copies/mL (range 417–5,130,000). Subjects were stratified by baseline HIV-1 RNA and CD4+ cell count. Forty-three percent of subjects had baseline viral loads >100,000 copies/mL and 39% had CD4+ cell counts <200 cells/mm3. Treatment outcomes through 48 and 144 weeks are presented in Table 17.
Table 17 Outcomes of Randomized Treatment at Week 48 and 144 (Study 903) At Week 48 At Week 144 Outcomes VIREAD+3TC
+EFV
(N=299)d4T+3TC
+EFV
(N=301)VIREAD+3TC
+EFV
(N=299)d4T+3TC
+EFV
(N=301)- *
- Subjects achieved and maintained confirmed HIV-1 RNA <400 copies/mL through Week 48 and 144.
- †
- Includes confirmed viral rebound and failure to achieve confirmed <400 copies/mL through Week 48 and 144.
- ‡
- Includes lost to follow-up, subject's withdrawal, noncompliance, protocol violation and other reasons.
Responder* 79% 82% 68% 62% Virologic failure† 6% 4% 10% 8% Rebound 5% 3% 8% 7% Never suppressed 0% 1% 0% 0% Added an antiretroviral agent 1% 1% 2% 1% Death <1% 1% <1% 2% Discontinued due to adverse event 6% 6% 8% 13% Discontinued for other reasons‡ 8% 7% 14% 15% Achievement of plasma HIV-1 RNA concentrations of less than 400 copies/mL at Week 144 was similar between the two treatment groups for the population stratified at baseline on the basis of HIV-1 RNA concentration (> or ≤100,000 copies/mL) and CD4+ cell count (< or ≥200 cells/mm3). Through 144 weeks of therapy, 62% and 58% of subjects in the VIREAD and stavudine arms, respectively achieved and maintained confirmed HIV-1 RNA <50 copies/mL. The mean increase from baseline in CD4+ cell count was 263 cells/mm3 for the VIREAD arm and 283 cells/mm3 for the stavudine arm.
Through 144 weeks, 11 subjects in the VIREAD group and 9 subjects in the stavudine group experienced a new CDC Class C event.
Study 934
Data through 144 weeks are reported for Study 934, a randomized, open-label, active-controlled multicenter trial comparing emtricitabine + VIREAD administered in combination with efavirenz versus zidovudine/lamivudine fixed-dose combination administered in combination with efavirenz in 511 antiretroviral-naïve subjects. From Weeks 96 to 144 of the trial, subjects received a fixed-dose combination of emtricitabine and tenofovir DF with efavirenz in place of emtricitabine + VIREAD with efavirenz. Subjects had a mean age of 38 years (range 18–80), 86% were male, 59% were Caucasian and 23% were Black. The mean baseline CD4+ cell count was 245 cells/mm3 (range 2–1191) and median baseline plasma HIV-1 RNA was 5.01 log10 copies/mL (range 3.56–6.54). Subjects were stratified by baseline CD4+ cell count (< or ≥200 cells/mm3); 41% had CD4+ cell counts <200 cells/mm3 and 51% of subjects had baseline viral loads >100,000 copies/mL. Treatment outcomes through 48 and 144 weeks for those subjects who did not have efavirenz resistance at baseline are presented in Table 18.
Table 18 Outcomes of Randomized Treatment at Week 48 and 144 (Study 934) Outcomes At Week 48 At Week 144 FTC
+VIREAD
+EFV
(N=244)AZT/3TC
+EFV
(N=243)FTC
+VIREAD
+EFV
(N=227)*AZT/3TC
+EFV
(N=229)*- *
- Subjects who were responders at Week 48 or Week 96 (HIV-1 RNA <400 copies/mL) but did not consent to continue the trial after Week 48 or Week 96 were excluded from analysis.
- †
- Subjects achieved and maintained confirmed HIV-1 RNA <400 copies/mL through Weeks 48 and 144.
- ‡
- Includes confirmed viral rebound and failure to achieve confirmed <400 copies/mL through Weeks 48 and 144.
- §
- Includes lost to follow-up, subject withdrawal, noncompliance, protocol violation and other reasons.
Responder† 84% 73% 71% 58% Virologic failure‡ 2% 4% 3% 6% Rebound 1% 3% 2% 5% Never suppressed 0% 0% 0% 0% Change in antiretroviral regimen 1% 1% 1% 1% Death <1% 1% 1% 1% Discontinued due to adverse event 4% 9% 5% 12% Discontinued for other reasons§ 10% 14% 20% 22% Through Week 48, 84% and 73% of subjects in the emtricitabine + VIREAD group and the zidovudine/lamivudine group, respectively, achieved and maintained HIV-1 RNA <400 copies/mL (71% and 58% through Week 144). The difference in the proportion of subjects who achieved and maintained HIV-1 RNA <400 copies/mL through 48 weeks largely results from the higher number of discontinuations due to adverse events and other reasons in the zidovudine/lamivudine group in this open-label trial. In addition, 80% and 70% of subjects in the emtricitabine + VIREAD group and the zidovudine/lamivudine group, respectively, achieved and maintained HIV-1 RNA <50 copies/mL through Week 48 (64% and 56% through Week 144). The mean increase from baseline in CD4+ cell count was 190 cells/mm3 in the EMTRIVA + VIREAD group and 158 cells/mm3 in the zidovudine/lamivudine group at Week 48 (312 and 271 cells/mm3 at Week 144).
Through 48 weeks, 7 subjects in the emtricitabine + VIREAD group and 5 subjects in the zidovudine/lamivudine group experienced a new CDC Class C event (10 and 6 subjects through 144 weeks).
Treatment-Experienced Adult Patients
Study 907
Study 907 was a 24-week, double-blind placebo-controlled multicenter trial of VIREAD added to a stable background regimen of antiretroviral agents in 550 treatment-experienced subjects. After 24 weeks of blinded trial treatment, all subjects continuing on trial were offered open-label VIREAD for an additional 24 weeks. Subjects had a mean baseline CD4+ cell count of 427 cells/mm3 (range 23–1385), median baseline plasma HIV-1 RNA of 2340 (range 50–75,000) copies/mL, and mean duration of prior HIV-1 treatment was 5.4 years. Mean age of the subjects was 42 years, 85% were male and 69% were Caucasian, 17% Black and 12% Hispanic.
The percent of subjects with HIV-1 RNA <400 copies/mL and outcomes of subjects through 48 weeks are summarized in Table 19.
Table 19 Outcomes of Randomized Treatment (Study 907) Outcomes 0–24 weeks 0–48 weeks 24–48 weeks VIREAD
(N=368)Placebo
(N=182)VIREAD
(N=368)Placebo Crossover
to VIREAD
(N=170)- *
- Subjects with HIV-1 RNA <400 copies/mL and no prior study drug discontinuation at Week 24 and 48 respectively.
- †
- Subjects with HIV-1 RNA ≥400 copies/mL efficacy failure or missing HIV-1 RNA at Week 24 and 48 respectively.
- ‡
- Includes lost to follow-up, subject withdrawal, noncompliance, protocol violation and other reasons.
HIV-1 RNA <400 copies/mL* 40% 11% 28% 30% Virologic failure† 53% 84% 61% 64% Discontinued due to adverse event 3% 3% 5% 5% Discontinued for other reasons‡ 3% 3% 5% 1% At 24 weeks of therapy, there was a higher proportion of subjects in the VIREAD arm compared to the placebo arm with HIV-1 RNA <50 copies/mL (19% and 1%, respectively). Mean change in absolute CD4+ cell counts by Week 24 was +11 cells/mm3 for the VIREAD group and -5 cells/mm3 for the placebo group. Mean change in absolute CD4+ cell counts by Week 48 was +4 cells/mm3 for the VIREAD group.
Through Week 24, one subject in the VIREAD group and no subjects in the placebo arm experienced a new CDC Class C event.
14.2 Clinical Efficacy in Adults with Chronic Hepatitis B
HBeAg-Negative Chronic Hepatitis B
Study 0102 was a Phase 3, randomized, double-blind, active-controlled trial of VIREAD 300 mg compared to HEPSERA 10 mg in 375 HBeAg- (anti-HBe+) subjects with compensated liver function, the majority of whom were nucleoside-naïve. The mean age of subjects was 44 years, 77% were male, 25% were Asian, 65% were Caucasian, 17% had previously received alpha-interferon therapy and 18% were nucleoside-experienced (16% had prior lamivudine experience). At baseline, subjects had a mean Knodell necroinflammatory score of 7.8; mean plasma HBV DNA was 6.9 log10 copies/mL; and mean serum ALT was 140 U/L.
HBeAg-Positive Chronic Hepatitis B
Study 0103 was a Phase 3, randomized, double-blind, active-controlled trial of VIREAD 300 mg compared to HEPSERA 10 mg in 266 HBeAg+ nucleoside-naïve subjects with compensated liver function. The mean age of subjects was 34 years, 69% were male, 36% were Asian, 52% were Caucasian, 16% had previously received alpha-interferon therapy, and <5% were nucleoside experienced. At baseline, subjects had a mean Knodell necroinflammatory score of 8.4; mean plasma HBV DNA was 8.7 log10 copies /mL; and mean serum ALT was 147 U/L.
The primary data analysis was conducted after all subjects reached 48 weeks of treatment and results are summarized below.
The primary efficacy endpoint in both trials was complete response to treatment defined as HBV DNA <400 copies/mL (69 IU/mL) and Knodell necroinflammatory score improvement of at least 2 points, without worsening in Knodell fibrosis at Week 48 (Table 20).
Table 20 Histological, Virological, Biochemical, and Serological Response at Week 48 0102 (HBeAg-) 0103 (HBeAg+) VIREAD
(N=250)HEPSERA
(N=125)VIREAD
(N=176)HEPSERA
(N=90)Complete Response 71% 49% 67% 12% Histology Histological Response* 72%
69% 74% 68% HBV DNA
<400 copies/mL (<69 IU/mL)
93%
63%
76%
13%ALT
Normalized ALT†76% 77% 68% 54% Serology
HBeAg Loss/Seroconversion
NA‡
NA‡
20%/19%
16%/16%HBsAg Loss/Seroconversion 0/0 0/0 3%/1% 0/0 Treatment Beyond 48 Weeks
In Studies 0102 (HBeAg-negative) and 0103 (HBeAg-positive), subjects who completed double-blind treatment (389 and 196 subjects who were originally randomized to VIREAD and HEPSERA, respectively) were eligible to roll over to open-label VIREAD with no interruption in treatment.
In Study 0102, 266 of 347 subjects who entered the open-label period (77%) continued in the study through Week 384. Among subjects randomized to VIREAD followed by open-label treatment with VIREAD, 73% had HBV DNA <400 copies/ml (69 IU/ml), and 63% had ALT normalization at Week 384. Among subjects randomized to HEPSERA followed by open-label treatment with VIREAD, 80% had HBV DNA <400 copies/mL (69 IU/mL) and 70% had ALT normalization through Week 384. At Week 384, both HBsAg loss and seroconversion were approximately 1% in both treatment groups.
In Study 0103, 146 of 238 subjects who entered the open-label period (61%) continued in the study through Week 384. Among subjects randomized to VIREAD, 49% had HBV DNA <400 copies/mL (69 IU/mL), 42% had ALT normalization, and 20% had HBeAg loss (13% seroconversion to anti-HBe antibody) through Week 384. Among subjects randomized to HEPSERA followed by open-label treatment with VIREAD, 56% had HBV DNA <400 copies/mL (69 IU/mL), 50% had ALT normalization, and 28% had HBeAg loss (19% seroconversion to anti-HBe antibody) through Week 384. At Week 384, HBsAg loss and seroconversion were 11% and 8% respectively, in subjects initially randomized to VIREAD and 12% and 10%, respectively, in subjects initially randomized to HEPSERA.
Of the originally randomized and treated 641 subjects in the two studies, liver biopsy data from 328 subjects who received continuing open-label treatment with VIREAD monotherapy were available for analysis at baseline, Week 48 and Week 240. There were no apparent differences between the subset of subjects who had liver biopsy data at Week 240 and those subjects remaining on open-label VIREAD without biopsy data that would be expected to affect histological outcomes at Week 240. Among the 328 subjects evaluated, the observed histological response rates were 80% and 88% at Week 48 and Week 240, respectively. In the subjects without cirrhosis at baseline (Ishak fibrosis score 0-4), 92% (216/235) and 95% (223/235) had either improvement or no change in Ishak fibrosis score at Week 48 and Week 240, respectively. In subjects with cirrhosis at baseline (Ishak fibrosis score 5-6), 97% (90/93) and 99% (92/93) had either improvement or no change in Ishak fibrosis score at Week 48 and Week 240, respectively. Twenty-nine percent (27/93) and 72% (67/93) of subjects with cirrhosis at baseline experienced regression of cirrhosis by Week 48 and Week 240, respectively, with a reduction in Ishak fibrosis score of at least 2 points. No definitive conclusions can be established about the remaining study population who were not part of this subset analysis.
Patients with Lamivudine-Resistant Chronic Hepatitis B
Study 121 was a randomized, double-blind, active-controlled trial evaluating the safety and efficacy of VIREAD compared to an unapproved antiviral regimen in subjects with chronic hepatitis B, persistent viremia (HBV DNA ≥ 1,000 IU/mL), and genotypic evidence of lamivudine resistance (rtM204I/V +/- rtL180M). One hundred forty-one adult subjects were randomized to the VIREAD treatment arm. The mean age of subjects randomized to VIREAD was 47 years (range 18–73), 74% were male, 59% were Caucasian, and 37% were Asian. At baseline, 54% of subjects were HBeAg-negative, 46% were HBeAg-positive, and 56% had abnormal ALT. Subjects had a mean HBV DNA of 6.4 log10 copies/mL and mean serum ALT of 71 U/L at baseline.
After 96 weeks of treatment, 126 of 141 subjects (89%) randomized to VIREAD had HBV DNA < 400 copies/mL (69 IU/mL), and 49 of 79 subjects (62%) with abnormal ALT at baseline had ALT normalization. Among the HBeAg-positive subjects randomized to VIREAD, 10 of 65 subjects (15%) experienced HBeAg loss, and 7 of 65 subjects (11%) experienced anti-HBe seroconversion through Week 96. The proportion of subjects with HBV DNA concentrations below 400 copies/mL (69 IU/mL) at Week 96 was similar between the VIREAD monotherapy and the comparator arms.
Across the combined chronic hepatitis B treatment trials, the number of subjects with adefovir-resistance associated substitutions at baseline was too small to establish efficacy in this subgroup.
Patients with Chronic Hepatitis B and Decompensated Liver Disease
VIREAD was studied in a small randomized, double-blind, active-controlled trial evaluating the safety of VIREAD compared to other antiviral drugs in subjects with chronic hepatitis B and decompensated liver disease through 48 weeks (Study 0108).
Forty-five adult subjects (37 males and 8 females) were randomized to the VIREAD treatment arm. At baseline, 69% subjects were HBeAg-negative, and 31% were HBeAg-positive. Subjects had a mean Child-Pugh score of 7, a mean MELD score of 12, mean HBV DNA of 5.8 log10 copies/mL and mean serum ALT of 61 U/L at baseline. Trial endpoints were discontinuation due to an adverse event and confirmed increase in serum creatinine ≥ 0.5 mg/dL or confirmed serum phosphorus of < 2 mg/dL [See Adverse Reactions (6.1)].
At 48 weeks, 31/44 (70%) and 12/26 (46%) Viread-treated subjects achieved an HBV DNA < 400 copies/mL (69 IU/mL), and normalized ALT, respectively. The trial was not designed to evaluate treatment impact on clinical endpoints such as progression of liver disease, need for liver transplantation, or death.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
VIREAD tablets, 300 mg, are almond-shaped, light blue, film-coated tablets containing 300 mg of tenofovir disoproxil fumarate, which is equivalent to 245 mg of tenofovir disoproxil, are debossed with "GILEAD" and "4331" on one side and with "300" on the other side. Each bottle contains 30 tablets, a desiccant (silica gel canister or sachet), and closed with a child-resistant closure. (NDC 61958-0401-1)
NDC 69189-0401-1 single dose pack with 1 tablet as repackaged by Avera McKennan Hospital
Oral Powder
VIREAD oral powder consists of white, coated granules containing 40 mg of tenofovir disoproxil fumarate, which is equivalent to 33 mg of tenofovir disoproxil, per gram of powder and is available in multi-use bottles containing 60 grams of oral powder, closed with a child-resistant closure, and co-packaged with a dosing scoop. (NDC 61958-0403-1)
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- VIREAD is not a cure for HIV-1 infection and patients may continue to experience illnesses associated with HIV-1 infection, including opportunistic infections. Patients should remain under the care of a physician when using VIREAD.
- Patients should avoid doing things that can spread HIV or HBV to others.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safer sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
- Do not breastfeed. Tenofovir is excreted in breast milk and it is not known whether it can harm the baby. Mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in the breast milk.
- The long-term effects of VIREAD are unknown.
- VIREAD tablets and oral powder are for oral ingestion only.
- VIREAD should not be discontinued without first informing their physician.
- If you have HIV-1 infection, with or without HBV coinfection, it is important to take VIREAD with combination therapy.
- It is important to take VIREAD on a regular dosing schedule and to avoid missing doses.
- Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported. Treatment with VIREAD should be suspended in any patient who develops clinical symptoms suggestive of lactic acidosis or pronounced hepatotoxicity (including nausea, vomiting, unusual or unexpected stomach discomfort, and weakness) [See Warnings and Precautions (5.1)].
- Severe acute exacerbations of hepatitis have been reported in patients who are infected with HBV or coinfected with HBV and HIV-1 and have discontinued VIREAD [See Warnings and Precautions (5.2)].
- Renal impairment, including cases of acute renal failure and Fanconi syndrome, has been reported. VIREAD should be avoided with concurrent or recent use of a nephrotoxic agent (e.g., high-dose or multiple NSAIDs) [See Warnings and Precautions (5.3)]. Dosing interval of VIREAD may need adjustment in patients with renal impairment [See Dosage and Administration (2.3)].
- VIREAD should not be coadministered with the fixed-dose combination products ATRIPLA, COMPLERA, STRIBILD, and TRUVADA since it is a component of these products [See Warnings and Precautions (5.4)].
- VIREAD should not be administered in combination with HEPSERA [See Warnings and Precautions (5.4)].
- Patients with HIV-1 should be tested for Hepatitis B virus (HBV) before initiating antiretroviral therapy [See Warnings and Precautions (5.5)].
- In patients with chronic hepatitis B, it is important to obtain HIV antibody testing prior to initiating VIREAD [See Warnings and Precautions (5.5)].
- Decreases in bone mineral density have been observed with the use of VIREAD. Bone mineral density monitoring should be considered in patients who have a history of pathologic bone fracture or at risk for osteopenia [See Warnings and Precautions (5.6)].
- In the treatment of chronic hepatitis B, the optimal duration of treatment is unknown. The relationship between response and long-term prevention of outcomes such as hepatocellular carcinoma is not known.
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
VIREAD® (VEER-ee-ad)
(tenofovir disoproxil fumarate)
tablets and oral powderRead this Patient Information before you start taking VIREAD and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about VIREAD?
VIREAD can cause serious side effects, including:
- 1.
-
Build-up of an acid in your blood (lactic acidosis). Lactic acidosis can happen in some people who take VIREAD. Lactic acidosis is a serious medical emergency that can lead to death.
Lactic acidosis can be hard to identify early, because the symptoms could seem like symptoms of other health problems. Call your healthcare provider right away if you get the following symptoms which could be signs of lactic acidosis:- feeling very weak or tired
- have unusual (not normal) muscle pain
- have trouble breathing
- have stomach pain with
- nausea (feel sick to your stomach)
- vomiting
- feel cold, especially in your arms and legs
- feel dizzy or lightheaded
- have a fast or irregular heartbeat
- 2.
-
Severe liver problems. Severe liver problems can happen in people who take VIREAD or similar medicines. In some cases these liver problems can lead to death. Your liver may become large (hepatomegaly) and you may develop fat in your liver (steatosis) when you take VIREAD.
Call your healthcare provider right away if you have any of the following symptoms of liver problems:- your skin or the white part of your eyes turns yellow (jaundice).
- dark "tea-colored" urine
- light-colored bowel movements (stools)
- loss of appetite for several days or longer
- nausea
- stomach pain
You may be more likely to get lactic acidosis or severe liver problems if you are female, very overweight (obese), or have been taking VIREAD for a long time.
- 3.
-
Worsening of your Hepatitis B infection. Your hepatitis B Virus (HBV) infection may become worse (flare-up) if you take VIREAD and then stop it. A "flare-up" is when your HBV infection suddenly returns in a worse way than before.
- Do not let your VIREAD run out. Refill your prescription or talk to your healthcare provider before your VIREAD is all gone.
- Do not stop taking VIREAD without first talking to your healthcare provider.
- If you stop taking VIREAD, your healthcare provider will need to check your health often and do blood tests regularly to check your HBV infection. Tell your healthcare provider about any new or unusual symptoms you may have after you stop taking VIREAD.
- 4.
- Talk to your doctor about taking an HIV test before starting treatment with VIREAD for chronic hepatitis B. You should also get a test for HBV if you are taking VIREAD for treatment of HIV.
What is VIREAD?
VIREAD is a prescription medicine used:
- 1.
- with other antiviral medicines to treat Human Immunodeficiency Virus-1 (HIV-1) in people 2 years of age and older. HIV is the virus that causes AIDS (Acquired Immune Deficiency Syndrome).
- When used with other HIV medicines, VIREAD may reduce the amount of HIV in your blood (called "viral load"). VIREAD may also help to increase the number of CD4 (T) cells in your blood which help fight off other infections. Reducing the amount of HIV and increasing the CD4 (T) cell count may improve your immune system. This may reduce your risk of death or infections that can happen when your immune system is weak (opportunistic infections).
- VIREAD does not cure HIV infection or AIDS. People taking VIREAD may still develop infections or other conditions associated with HIV infection.
- You must stay on continuous HIV therapy to control infection and decrease HIV-related illnesses.
- It is very important that you stay under the care of your healthcare provider.
- It is not known if VIREAD is safe and effective for the treatment of HIV-1 infection in children under the age of 2 years.
- 2.
- to treat chronic (long-lasting) hepatitis B virus (HBV) in people 12 years of age and older.
- VIREAD will not cure HBV.
- VIREAD may lower the amount of HBV in your body.
- VIREAD may improve the condition of your liver.
- The long-term effects of taking VIREAD for treatment of chronic hepatitis B infection are not known.
- It is not known if VIREAD is safe and effective for treatment of chronic hepatitis B in children under the age of 12 years.
What should I tell my healthcare provider before taking VIREAD?
Before you take VIREAD, tell your healthcare provider if you:
- have liver problems, including hepatitis B (HBV) infection.
- have kidney problems.
- have bone problems.
- have any other medical conditions, including HIV infection.
- are pregnant or plan to become pregnant. It is not known if VIREAD will harm your unborn baby.
Pregnancy Registry. There is a pregnancy registry for women who take antiviral medicines during pregnancy. Its purpose is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry. - are breastfeeding or plan to breastfeed. Do not breastfeed if you are taking VIREAD. Tenofovir passes into your breast milk. You should not breastfeed because of the risk of passing HIV to your baby. Talk to your healthcare provider about the best way to feed your baby.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. VIREAD may affect the way other medicines work, and other medicines may affect how VIREAD works.
Do not take VIREAD if you also take:
- other medicines that contain tenofovir (ATRIPLA®, COMPLERA®, STRIBILD®, TRUVADA®)
- adefovir (HEPSERA®)
Especially tell your healthcare provider if you take the following medications.
- didanosine (Videx, Videx EC)
- atazanavir (Reyataz)
- darunavir (Prezista)
- lopinavir with ritonavir (Kaletra)
Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How should I take VIREAD?
- See "What is the most important information I should know about VIREAD?"
- Take VIREAD exactly as your healthcare provider tells you to take it.
- Take VIREAD at the same time every day.
- For adults and children 12 years of age and older, the usual dose of VIREAD is one 300 mg tablet each day.
- If you are an adult with kidney problems, your healthcare provider may tell you to take VIREAD less often.
- Adults and children 12 years of age and older who are unable to swallow VIREAD tablets whole may take 7½ scoops of VIREAD oral powder.
- For children 2 to 12 years of age, your healthcare provider will prescribe the right dose of VIREAD oral powder or tablets based on your child's body weight.
- Tell your healthcare provider if your child has problems with swallowing tablets.
- See the "Instructions for Use" section at the end of this Patient Information leaflet for information about the right way to measure and take VIREAD oral powder.
- Take VIREAD tablets by mouth, with or without food.
- Do not miss a dose of VIREAD. If you miss a dose of VIREAD, take the missed dose as soon as you remember. If it is almost time for your next dose of VIREAD, do not take the missed dose. Take the next dose of VIREAD at your regular time.
- If you take too much VIREAD, call your local poison control center or go right away to the nearest hospital emergency room.
What are the possible side effects of VIREAD?
VIREAD may cause serious side effects, including:
- See "What is the most important information I should know about VIREAD?"
- New or worse kidney problems, including kidney failure, can happen in some people who take VIREAD. Your healthcare provider should do blood tests to check your kidneys before you start treatment with VIREAD. If you have had kidney problems in the past or need to take another medicine that can cause kidney problems, your healthcare provider may need to do blood tests to check your kidneys during your treatment with VIREAD.
- Bone problems can happen in some people who take VIREAD. Bone problems include bone pain, softening or thinning (which may lead to fractures). Your healthcare provider may need to do additional tests to check your bones.
- Changes in body fat can happen in some people who take antiviral medicines. These changes may include increased amount of fat in the upper back and neck ("buffalo hump"), breast, and around the main part of your body (trunk). Loss of fat from the legs, arms, and face may also happen. The cause and long-term health effects of these conditions are not known.
- Changes in your immune system (Immune Reconstitution Syndrome) can happen when you start taking HIV medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider if you start having new symptoms after starting your HIV medicine.
The most common side effects in all people who take VIREAD are:
- nausea
- rash
- diarrhea
- headache
- pain
- depression
- weakness
In some people with advanced HBV-infection, other common side effects may include:
- sleeping problems
- itching
- vomiting
- dizziness
- fever
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of VIREAD. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
- Store VIREAD tablets or oral powder at room temperature between 68 °F to 77 °F (20 °C to 25 °C).
- Keep VIREAD in the original container.
- Do not use VIREAD if the seal over the bottle opening is broken or missing.
- Keep the bottle tightly closed.
Keep VIREAD and all medicines out of the reach of children.
General information about VIREAD:
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use VIREAD for a condition for which it was not prescribed. Do not give VIREAD to other people, even if they have the same condition you have. It may harm them.
Avoid doing things that can spread HIV-1 or HBV infection to others.
- Do not share or re-use needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
A vaccine is available to protect people at risk for becoming infected with HBV. You can ask your healthcare provider for information about this vaccine.
This leaflet summarizes the most important information about VIREAD. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about VIREAD that is written for health professionals.
For more information, go to www.viread.com or call Gilead Sciences, Inc. at 1-800-GILEAD-5 (1-800-445-3235).
What are the ingredients in VIREAD?
Active Ingredient: tenofovir disoproxil fumarate
Inactive Ingredients:
VIREAD tablets: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and pregelatinized starch.
VIREAD Oral Powder: mannitol, hydroxypropyl cellulose, ethylcellulose, and silicon dioxide.
Tablet Coating:
VIREAD tablets 300 mg: Opadry II Y-30-10671-A, which contains FD&C blue #2 aluminum lake, hypromellose 2910, lactose monohydrate, titanium dioxide, and triacetin.
VIREAD tablets 150, 200 and 250 mg: Opadry II 32K-18425, which contains hypromellose 2910, lactose monohydrate, titanium dioxide, and triacetin.
-
SPL UNCLASSIFIED SECTION
Manufactured for and distributed by:
Gilead Sciences, Inc.
Foster City, CA 94404Revised: May 2015
COMPLERA, EMTRIVA, GSI, HEPSERA, STRIBILD, TRUVADA, and VIREAD are trademarks or registered trademarks of Gilead Sciences, Inc., or its related companies. ATRIPLA is a registered trademark of Bristol-Myers Squibb & Gilead Sciences, LLC. All other trademarks herein are the property of their respective owners.
21-356-GS-036
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VIREAD
tenofovir disoproxil fumarate tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69189-0401(NDC:61958-0401) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TENOFOVIR DISOPROXIL FUMARATE (UNII: OTT9J7900I) (TENOFOVIR ANHYDROUS - UNII:W4HFE001U5) TENOFOVIR DISOPROXIL FUMARATE 300 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) HYPROMELLOSE 2910 (15000 MPA.S) (UNII: 288VBX44JC) Product Characteristics Color BLUE (LIGHT BLUE) Score no score Shape FREEFORM (Almond-shaped) Size 17mm Flavor Imprint Code GILEAD;4331;300 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69189-0401-1 1 in 1 DOSE PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021356 05/18/2015 Labeler - Avera McKennan Hospital (068647668) Establishment Name Address ID/FEI Business Operations Avera McKennan Hospital 068647668 relabel(69189-0401) , repack(69189-0401)


