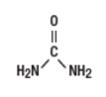
Label: UREA 40 PERCENT- urea cream
- NDC Code(s): 58657-487-01, 58657-487-03, 58657-487-07
- Packager: Method Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION:
- CLINICAL PHARMACOLOGY:
- INDICATIONS:
- CONTRAINDICATIONS:
-
WARNINGS:
FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE. KEEP OUT OF REACH OF CHILDREN.
Avoid contact with eyes, lips and mucous membranes.
General:
This product is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use and consult a physician.
Information for Patients:
Patients should discontinue the use of this product if the condition becomes worse or if a rash develops in the area being treated or elsewhere. Avoid contact with eyes, lips and mucous membranes.
Carcinogenesis, Mutagenesis and Impairment of Fertility:
Long-term animal studies for carcinogenic potential have not been performed on this product to date. Studies on reproduction and fertility also have not been performed.
Pregnancy:
Category C. Animal reproduction studies have not been conducted with this product. It is also not known whether this product can affect reproduction capacity or cause fetal harm when administered to a pregnant woman. This product should be used by a pregnant woman only if clearly needed or when potential benefits outweigh potential hazards to the fetus.
- ADVERSE REACTIONS:
- DOSAGE AND ADMINISTRATION:
- STORAGE:
-
HOW SUPPLIED:
This product is supplied in the following size(s): 3 oz, 1 oz, and 7oz
To report a serious adverse event or obtain product information, call 877-250-3427
To report a serious adverse event, please contact Method Pharmaceuticals at (877) 250-3427; email at contact@methodpharm.com; or call FDA at (800) FDA-1088.
Manufactured for:
Method Pharmaceuticals, LLC Fort Worth, Texas 76118
Rev. 11/21 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UREA 40 PERCENT
urea creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:58657-487 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UREA (UNII: 8W8T17847W) (UREA - UNII:8W8T17847W) UREA 400 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) CETYL ALCOHOL (UNII: 936JST6JCN) WHITE PETROLATUM (UNII: B6E5W8RQJ4) MINERAL OIL (UNII: T5L8T28FGP) WATER (UNII: 059QF0KO0R) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58657-487-07 198.4 g in 1 BOTTLE; Type 0: Not a Combination Product 11/15/2021 2 NDC:58657-487-01 28.35 g in 1 BOTTLE; Type 0: Not a Combination Product 11/15/2021 3 NDC:58657-487-03 85 g in 1 BOTTLE; Type 0: Not a Combination Product 11/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/15/2021 Labeler - Method Pharmaceuticals, LLC (060216698)