Label: LABETALOL HYDROCHLORIDE injection, solution
- NDC Code(s): 0409-2339-24, 0409-2339-34
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 28, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Labetalol hydrochloride is an adrenergic receptor blocking agent that has both selective alpha1- and nonselective beta-adrenergic receptor blocking actions in a single substance.
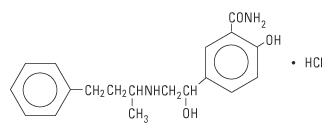
Labetalol HCl is a racemate, chemically designated as 5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl) amino] ethyl]-salicylamide monohydrochloride, and has the following structural formula:
Labetalol hydrochloride has the molecular formula C19H24N2O3 • HCl and a molecular weight of 364.87. It has two asymmetric centers and therefore exists as a molecular complex of two diastereoisomeric pairs. Dilevalol, the R,R' stereoisomer, makes up 25% of racemic labetalol.
Labetalol hydrochloride is a white or off-white crystalline powder, soluble in water.
Labetalol hydrochloride injection is a clear, colorless to light yellow aqueous sterile isotonic solution for intravenous injection. It has a pH range of 3.0 to 4.5. Each mL contains 5 mg labetalol hydrochloride, USP, 45 mg anhydrous dextrose, 0.1 mg edetate disodium; 0.8 mg methylparaben and 0.1 mg propylparaben as preservatives; citric acid monohydrate and sodium hydroxide, as necessary, to bring the solution into the pH range.
-
CLINICAL PHARMACOLOGY
Labetalol combines both selective, competitive alpha1-adrenergic blocking and nonselective, competitive beta-adrenergic blocking activity in a single substance. In man, the ratios of alpha- to beta-blockade have been estimated to be approximately 1:3 and 1:7 following oral and intravenous administration, respectively. Beta2-agonist activity has been demonstrated in animals with minimal beta1-agonist (ISA) activity detected. In animals, at doses greater than those required for alpha- or beta-adrenergic blockade, a membrane-stabilizing effect has been demonstrated.
Pharmacodynamics
The capacity of labetalol to block alpha-receptors in man has been demonstrated by attenuation of the presser effect of phenylephrine and by a significant reduction of the presser response caused by immersing the hand in ice-cold water ("cold-presser test"). Labetalol's beta1-receptor blockade in man was demonstrated by a small decrease in the resting heart rate, attenuation of tachycardia produced by isoproterenol or exercise, and by attenuation of the reflex tachycardia to the hypotension produced by amyl nitrite. Beta2-receptor blockade was demonstrated by inhibition of the isoproterenol-induced fall in diastolic blood pressure. Both the alpha- and beta-blocking actions of orally administered labetalol contribute to a decrease in blood pressure in hypertensive patients. Labetalol consistently, in dose-related fashion, blunted increases in exercise-induced blood pressure and heart rate, and in their double product. The pulmonary circulation during exercise was not affected by labetalol dosing.
Single oral doses of labetalol administered in patients with coronary artery disease had no significant effect on sinus rate, intraventricular conduction, or QRS duration. The atrioventricular (AV) conduction time was modestly prolonged in 2 of 7 patients. In another study, intravenous labetalol slightly prolonged AV nodal conduction time and atrial effective refractory period with only small changes in heart rate. The effects on AV nodal refractoriness were inconsistent.
Labetalol produces dose-related falls in blood pressure without reflex tachycardia and without significant reduction in heart rate, presumably through a mixture of its alpha-blocking and beta-blocking effects. Hemodynamic effects are variable with small nonsignificant changes in cardiac output seen in some studies but not others, and small decreases in total peripheral resistance. Elevated plasma renins are reduced.
Doses of labetalol that controlled hypertension did not affect renal function in mild to severe hypertensive patients with normal renal function.
Due to the alpha1-receptor blocking activity of labetalol, blood pressure is lowered more in the standing than in the supine position, and symptoms of postural hypotension can occur. During dosing with intravenous labetalol, the contribution of the postural component should be considered when positioning patients for treatment, and patients should not be allowed to move to an erect position unmonitored until their ability to do so is established.
In a clinical pharmacologic study in severe hypertensives, an initial 0.25 mg/kg injection of labetalol, administered to patients in the supine position, decreased blood pressure by an average of 11/7 mmHg. Additional injections of 0.5 mg/kg at 15 minute intervals up to a total cumulative dose of 1.75 mg/kg of labetalol caused further dose-related decreases in blood pressure. Some patients required cumulative doses of up to 3.25 mg/kg. The maximal effect of each dose level occurred within 5 minutes. Following discontinuation of intravenous treatment with labetalol, the blood pressure rose gradually and progressively approaching pretreatment baseline values within an average of 16 to 18 hours in the majority of patients.
Similar results were obtained in the treatment of patients with severe hypertension requiring urgent blood pressure reduction with an initial dose of 20 mg (which corresponds to 0.25 mg/kg for an 80 kg patient) followed by additional doses of either 40 or 80 mg at 10 minute intervals to achieve the desired effect or up to a cumulative dose of 300 mg.
Labetalol administered as a continuous intravenous infusion, with a mean dose of 136 mg (27 to 300 mg) over a period of 2 to 3 hours (mean of 2 hours and 39 minutes) lowered the blood pressure by an average of 60/35 mmHg.
Exacerbation of angina and, in some cases, myocardial infarction and ventricular dysrhythmias have been reported after abrupt discontinuation of therapy with beta-adrenergic blocking agents in patients with coronary artery disease. Abrupt withdrawal of these agents in patients without coronary artery disease has resulted in transient symptoms, including tremulousness, sweating, palpitation, headache and malaise. Several mechanisms have been proposed to explain these phenomena, among them increased sensitivity to catecholamines because of increased numbers of beta-receptors.
Although beta-adrenergic receptor blockade is useful in the treatment of angina and hypertension, there are also situations in which sympathetic stimulation is vital. For example, in patients with severely damaged hearts, adequate ventricular function may depend on sympathetic drive. Beta-adrenergic blockade may worsen AV block by preventing the necessary facilitating effects of sympathetic activity on conduction. Beta2-adrenergic blockade results in passive bronchial constriction by interfering with endogenous adrenergic bronchodilator activity in patients subject to bronchospasm and may also interfere with exogenous bronchodilators in such patients.
Pharmacokinetics and Metabolism
Following intravenous infusion, the elimination half-life is about 5.5 hours and the total body clearance is approximately 33 mL/min/kg. The plasma half-life of labetalol following oral administration is about 6 to 8 hours. In patients with decreased hepatic or renal function, the elimination half-life of labetalol is not altered; however, the relative bioavailability in hepatically impaired patients is increased due to decreased "first-pass" metabolism.
The metabolism of labetalol is mainly through conjugation to glucuronide metabolites. These metabolites are present in plasma and are excreted in the urine and, via the bile, into the feces. Approximately 55% to 60% of a dose appears in the urine as conjugates or unchanged labetalol within the first 24 hours of dosing.
Labetalol has been shown to cross the placental barrier in humans. Only negligible amounts of the drug crossed the blood-brain barrier in animal studies. Labetalol is approximately 50% protein bound. Neither hemodialysis nor peritoneal dialysis removes a significant amount of labetalol from the general circulation (<1%).
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Labetalol HCl injection is contraindicated in bronchial asthma, overt cardiac failure, greater than first degree heart block, cardiogenic shock, severe bradycardia, other conditions associated with severe and prolonged hypotension, and in patients with a history of hypersensitivity to any component of the product (see WARNINGS).
Beta-blockers, even those with apparent cardioselectivity, should not be used in patients with a history of obstructive airway disease, including asthma.
-
WARNINGS
Hepatic Injury
Severe hepatocellular injury, confirmed by rechallenge in at least one case, occurs rarely with labetalol therapy. The hepatic injury is usually reversible, but hepatic necrosis and death have been reported. Injury has occurred after both short- and long-term treatment and may be slowly progressive despite minimal symptomatology. Similar hepatic events have been reported with a related compound, dilevalol HCl, including two deaths. Dilevalol HCl is one of the four isomers of labetalol. Thus, for patients taking labetalol, periodic determination of suitable hepatic laboratory tests would be appropriate. Laboratory testing should also be done at the very first symptom or sign of liver dysfunction (e.g., pruritus, dark urine, persistent anorexia, jaundice, right upper quadrant tenderness, or unexplained "flu-like" symptoms). If the patient has jaundice or laboratory evidence of liver injury, labetalol should be stopped and not restarted.
Cardiac Failure
Sympathetic stimulation is a vital component supporting circulatory function in congestive heart failure. Beta-blockade carries a potential hazard of further depressing myocardial contractility and precipitating more severe failure. Although beta-blockers should be avoided in overt congestive heart failure, if necessary, labetalol can be used with caution in patients with a history of heart failure, who are well compensated. Congestive heart failure has been observed in patients receiving labetalol. Labetalol does not abolish the inotropic action of digitalis on heart muscle.
In Patients without a History of Cardiac Failure
In patients with latent cardiac insufficiency, continued depression of the myocardium with beta-blocking agents over a period of time can lead, in some cases, to cardiac failure. At the first sign or symptom of impending cardiac failure, patients should be fully digitalized and/or be given a diuretic, and the response observed closely. If cardiac failure continues, despite adequate digitalization and diuretic, labetalol therapy should be withdrawn (gradually if possible).
Ischemic Heart Disease
Angina pectoris has not been reported upon labetalol discontinuation. However, following abrupt cessation of therapy with some beta-blocking agents in patients with coronary artery disease, exacerbations of angina pectoris and, in some cases, myocardial infarction have been reported. Therefore, such patients should be cautioned against interruption of therapy without the physician's advice. Even in the absence of overt angina pectoris, when discontinuation of labetalol is planned, the patient should be carefully observed and should be advised to limit physical activity. If angina markedly worsens or acute coronary insufficiency develops, labetalol administration should be reinstituted promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken.
Nonallergic Bronchospasm (e.g., chronic bronchitis and emphysema)
Since labetalol injection at the usual intravenous therapeutic doses has not been studied in patients with nonallergic bronchospastic disease, it should not be used in such patients.
Pheochromocytoma
Intravenous labetalol has been shown to be effective in lowering the blood pressure and relieving symptoms in patients with pheochromocytoma; higher than usual doses may be required. However, paradoxical hypertensive responses have been reported in a few patients with this tumor; therefore, use caution when administering labetalol to patients with pheochromocytoma.
Diabetes Mellitus and Hypoglycemia
Beta-adrenergic blockade may prevent the appearance of premonitory signs and symptoms (e.g., tachycardia) of acute hypoglycemia. This is especially important with labile diabetics. Beta-blockade also reduces the release of insulin in response to hyperglycemia; it may therefore be necessary to adjust the dose of antidiabetic drugs.
Major Surgery
Do not routinely withdraw chronic beta blocker therapy prior to surgery. The effect of labetalol's alpha adrenergic activity has not been evaluated in this setting.
Several deaths have occurred when labetalol injection was used during surgery (including when used in cases to control bleeding).
A synergism between labetalol and halothane anesthesia has been shown (see PRECAUTIONS - Drug Interactions).
Rapid Decreases of Blood Pressure
Caution must be observed when reducing severely elevated blood pressure. A number of adverse reactions, including cerebral infarction, optic nerve infarction, angina, and ischemic changes in the electrocardiogram, have been reported with other agents when severely elevated blood pressure was reduced over time courses of several hours to as long as 1 or 2 days. The desired blood pressure lowering should therefore be achieved over as long a period of time as is compatible with the patient's status.
-
PRECAUTIONS
General
Labetalol injection should be used with caution in patients with impaired hepatic function since metabolism of the drug may be diminished.
Hypotension
Symptomatic postural hypotension (incidence 58%) is likely to occur if patients are tilted or allowed to assume the upright position within 3 hours of receiving labetalol injection. Therefore, the patient's ability to tolerate an upright position should be established before permitting any ambulation.
Intraoperative Floppy Iris Syndrome (IFIS) has been observed during cataract surgery in some patients treated with alpha-1 blockers (labetalol is an alpha/beta blocker). This variant of small pupil syndrome is characterized by the combination of a flaccid iris that billows in response to intraoperative irrigation currents, progressive intraoperative miosis despite preoperative dilation with standard mydriatic drugs, and potential prolapse of the iris toward the phacoemulsification incisions. The patient's ophthalmologist should be prepared for possible modifications to the surgical technique, such as the utilization of iris hooks, iris dilator rings, or viscoelastic substances. There does not appear to be a benefit of stopping alpha-1 blocker therapy prior to cataract surgery.
Following Coronary Artery Bypass Surgery
In one uncontrolled study, patients with low cardiac indices and elevated systemic vascular resistance following intravenous labetalol experienced significant declines in cardiac output with little change in systemic vascular resistance. One of these patients developed hypotension following labetalol treatment. Therefore, use of labetalol should be avoided in such patients.
High-Dose Labetalol
Administration of up to 3 g/d as an infusion for up to 2 to 3 days has been anecdotally reported; several patients experienced hypotension or bradycardia.
Jaundice or Hepatic Dysfunction
(see WARNINGS).
Information for Patients
The following information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects. During and immediately following (for up to 3 hours) labetalol injection, the patient should remain supine. Subsequently, the patient should be advised on how to proceed gradually to become ambulatory, and should be observed at the time of first ambulation.
When the patient is started on labetalol hydrochloride tablets following adequate control of blood pressure with labetalol hydrochloride injection, appropriate directions for titration of dosage should be provided (see DOSAGE AND ADMINISTRATION).
As with all drugs with beta-blocking activity, certain advice to patients being treated with labetalol is warranted: While no incident of the abrupt withdrawal phenomenon (exacerbation of angina pectoris) has been reported with labetalol, dosing with labetalol tablets should not be interrupted or discontinued without a physician's advice. Patients being treated with labetalol tablets should consult a physician at any signs or symptoms of impending cardiac failure or hepatic dysfunction (see WARNINGS). Also, transient scalp tingling may occur, usually when treatment with labetalol tablets is initiated (see ADVERSE REACTIONS).
Laboratory Tests
Routine laboratory tests are ordinarily not required before or after intravenous labetalol. In patients with concomitant illnesses, such as impaired renal function, appropriate tests should be done to monitor these conditions.
Drug Interactions
Since labetalol injection may be administered to patients already being treated with other medications, including other antihypertensive agents, careful monitoring of these patients is necessary to detect and treat promptly any undesired effect from concomitant administration.
In one survey, 2.3% of patients taking labetalol orally in combination with tricyclic antidepressants experienced tremor as compared to 0.7% reported to occur with labetalol alone. The contribution of each of the treatments to this adverse reaction is unknown but the possibility of a drug interaction cannot be excluded.
Drugs possessing beta-blocking properties can blunt the bronchodilator effect of beta-receptor agonist drugs in patients with bronchospasm; therefore, doses greater than the normal antiasthmatic dose of beta-agonist bronchodilator drugs may be required.
Cimetidine has been shown to increase the bioavailability of labetalol administered orally. Since this could be explained either by enhanced absorption or by an alteration of hepatic metabolism of labetalol, special care should be used in establishing the dose required for blood pressure control in such patients.
Synergism has been shown between halothane anesthesia and intravenously administered labetalol. During controlled hypotensive anesthesia using labetalol in association with halothane, high concentrations (3% or above) of halothane should not be used because the degree of hypotension will be increased and because of the possibility of a large reduction in cardiac output and an increase in central venous pressure. The anesthesiologist should be informed when a patient is receiving labetalol.
Labetalol blunts the reflex tachycardia produced by nitroglycerin without preventing its hypotensive effect. If labetalol is used with nitroglycerin in patients with angina pectoris, additional antihypertensive effects may occur.
Care should be taken if labetalol is used concomitantly with calcium antagonists of the verapamil type.
When drug products that are alkaline, such as furosemide, have been administered in combination with labetalol, a white precipitate has been noted. Therefore, these drugs should not be administered in the same infusion line.
Risk of Anaphylactic Reaction
While taking beta-blockers, patients with a history of severe anaphylactic reactions to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
Drug/Laboratory Test Interactions
The presence of labetalol metabolites in the urine may result in falsely elevated levels of urinary catecholamines, metanephrine, normetanephrine, and vanillylmandelic acid (VMA) when measured by fluorimetric or photometric methods. In screening patients suspected of having a pheochromocytoma and being treated with labetalol, a specific method, such as a high-performance liquid chromatographic assay with solid phase extraction (e.g., J Chromatogr. 385:241, 1987) should be employed in determining levels of catecholamines.
Labetalol has also been reported to produce a false-positive test for amphetamine when screening urine for the presence of drugs using the commercially available assay methods Toxi-Lab A® (thin-layer chromatographic assay) and Emit-d.a.u.® (radioenzymatic assay). When patients being treated with labetalol have a positive urine test for amphetamine using these techniques, confirmation should be made by using more specific methods, such as a gas chromatographic-mass spectrometer technique.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term oral dosing studies with labetalol for 18 months in mice and for 2 years in rats showed no evidence of carcinogenesis. Studies with labetalol, using dominant lethal assays in rats and mice, and exposing microorganisms according to modified Ames tests, showed no evidence of mutagenesis.
Pregnancy
Teratogenic Effects
Teratogenic studies have been performed with labetalol in rats and rabbits at oral doses up to approximately 6 and 4 times the maximum recommended human dose (MRHD), respectively. No reproducible evidence of fetal malformations was observed. Increased fetal resorptions were seen in both species at doses approximating the MRHD. A teratology study performed with labetalol in rabbits at intravenous doses up to 1.7 times the MRHD revealed no evidence of drug-related harm to the fetus. There are no adequate and well-controlled studies in pregnant women. Labetalol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
Hypotension, bradycardia, hypoglycemia, and respiratory depression have been reported in infants of mothers who were treated with labetalol for hypertension during pregnancy. Oral administration of labetalol to rats during late gestation through weaning at doses of 2 to 4 times the MRHD caused a decrease in neonatal survival.
Labor and Delivery
Labetalol given to pregnant women with hypertension did not appear to affect the usual course of labor and delivery.
Nursing Mothers
Small amounts of labetalol (approximately 0.004% of the maternal dose) are excreted in human milk. Caution should be exercised when labetalol injection is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in children have not been established.
-
ADVERSE REACTIONS
Labetalol injection is usually well tolerated. Most adverse effects have been mild and transient and in controlled trials involving 92 patients did not require labetalol withdrawal. Symptomatic postural hypotension (incidence 58%) is likely to occur if patients are tilted or allowed to assume the upright position within 3 hours of receiving labetalol injection. Moderate hypotension occurred in 1 of 100 patients while supine. Increased sweating was noted in 4 of 100 patients, and flushing occurred in 1 of 100 patients.
The following also were reported with labetalol injection with the incidence per 100 patients as noted:
Cardiovascular System: Ventricular arrhythmia in 1.
Central and Peripheral Nervous Systems: Dizziness in 9; tingling of the scalp/skin 7; hypoesthesia (numbness) and vertigo, 1 each.
Gastrointestinal System: Nausea in 13; vomiting 4; dyspepsia and taste distortion, 1 each.
Metabolic Disorders: Transient increases in blood urea nitrogen and serum creatinine levels occurred in 8 of 100 patients; these were associated with drops in blood pressure, generally in patients with prior renal insufficiency.
Psychiatric Disorders: Somnolence/yawning in 3.
Respiratory System: Wheezing in 1.
Skin: Pruritus in 1.
The incidence of adverse reactions depends upon the dose of labetalol. The largest experience is with oral labetalol. Certain of the side effects increased with increasing oral dose as shown in the table below which depicts the entire U.S. therapeutic trials data base for adverse reactions that are clearly or possibly dose related.
Labetalol Daily Dose (mg)
200
300
400
600
800
900
1200
1600
2400
Number of Patients
522
181
606
608
503
117
411
242
175
Dizziness (%)
2
3
3
3
5
1
9
13
16
Fatigue
2
1
4
4
5
3
7
6
10
Nausea
<1
0
1
2
4
0
7
11
19
Vomiting
0
0
<1
<1
<1
0
1
2
3
Dyspepsia
1
0
2
1
1
0
2
2
4
Paresthesias
2
0
2
2
1
1
2
5
5
Nasal Stuffiness
1
1
2
2
2
2
4
5
6
Ejaculation Failure
0
2
1
2
3
0
4
3
5
Impotence
1
1
1
1
2
4
3
4
3
Edema
1
0
1
1
1
0
1
2
2
In addition, a number of other less common adverse events have been reported:
Cardiovascular: Hypotension, and rarely, syncope, bradycardia, heart block.
Liver and Biliary System: Hepatic necrosis, hepatitis, cholestatic jaundice, elevated liver function tests.
Hypersensitivity: Rare reports of hypersensitivity (e.g., rash, urticaria, pruritus, angioedema, dyspnea) and anaphylactoid reactions.
The oculomucocutaneous syndrome associated with the beta-blocker practolol has not been reported with labetalol during investigational use and extensive foreign marketing experience.
Clinical Laboratory Tests
Among patients dosed with labetalol tablets, there have been reversible increases of serum transaminases in 4% of patients tested, and more rarely, reversible increases in blood urea.
-
OVERDOSAGE
Overdosage with labetalol injection causes excessive hypotension that is posture sensitive, and sometimes, excessive bradycardia. Patients should be placed supine and their legs raised if necessary to improve the blood supply to the brain. If overdosage with labetalol follows oral ingestion, gastric lavage or pharmacologically induced emesis (using syrup of ipecac) may be useful for removal of the drug shortly after ingestion. The following additional measures should be employed if necessary: Excessive bradycardia-administer atropine or epinephrine. Cardiac failure-administer a digitalis glycoside and a diuretic. Dopamine or dobutamine may also be useful. Hypotension-administer vasopressors, e.g., norepinephrine. There is pharmacological evidence that norepinephrine may be the drug of choice. Bronchospasm-administer epinephrine and/or an aerosolized beta2-agonist. Seizures-administer diazepam.
In severe beta-blocker overdose resulting in hypotension and/or bradycardia, glucagon has been shown to be effective when administered in large doses (5 to 10 mg rapidly over 30 seconds, followed by continuous infusion of 5 mg/hr that can be reduced as the patient improves).
Neither hemodialysis nor peritoneal dialysis removes a significant amount of labetalol from the general circulation (<1%).
The oral LD50 value of labetalol in the mouse is approximately 600 mg/kg and in the rat is greater than 2 g/kg. The intravenous LD50 in these species is 50 to 60 mg/kg.
-
DOSAGE AND ADMINISTRATION
Labetalol hydrochloride injection is intended for intravenous use in hospitalized patients. DOSAGE MUST BE INDIVIDUALIZED depending upon the severity of hypertension and the response of the patient during dosing.
Patients should always be kept in a supine position during the period of intravenous drug administration. A substantial fall in blood pressure on standing should be expected in these patients. The patient's ability to tolerate an upright position should be established before permitting any ambulation, such as using toilet facilities.
Either of two methods of administration of labetalol hydrochloride injection may be used: a) repeated intravenous injections, b) slow continuous infusion.
Repeated Intravenous Injection
Initially, labetalol hydrochloride injection should be given in a dose of 20 mg labetalol HCl (which corresponds to 0.25 mg/kg for an 80 kg patient) by slow intravenous injection over a 2-minute period.
Immediately before the injection and at 5 and 10 minutes after injection, supine blood pressure should be measured to evaluate response. Additional injections of 40 mg or 80 mg can be given at 10 minute intervals until a desired supine blood pressure is achieved or a total of 300 mg labetalol HCl has been injected. The maximum effect usually occurs within 5 minutes of each injection.
Slow Continuous Infusion
Labetalol hydrochloride injection is prepared for continuous intravenous infusion by diluting the drug with commonly used intravenous fluids (see below). Examples of methods of preparing the infusion solution are:
Labetalol hydrochloride injection 200 mg is added to 160 mL of a commonly used intravenous fluid such that the resultant 200 mL of solution contains 200 mg of labetalol hydrochloride, 1 mg/mL. The diluted solution should be administered at a rate of 2 mL/min to deliver 2 mg/min.
Alternatively, 200 mg of labetalol hydrochloride injection is added to 250 mL of a commonly used intravenous fluid. The resultant solution will contain 200 mg of labetalol hydrochloride, approximately 2 mg/3 mL. The diluted solution should be administered at a rate of 3 mL/min to deliver approximately 2 mg/min.
The rate of infusion of the diluted solution may be adjusted according to the blood pressure response, at the discretion of the physician. To facilitate a desired rate of infusion, the diluted solution can be infused using a controlled administration mechanism, e.g., graduated burette or mechanically driven infusion pump.
Since the half-life of labetalol is 5 to 8 hours, steady-state blood levels (in the face of a constant rate of infusion) would not be reached during the usual infusion time period. The infusion should be continued until a satisfactory response is obtained and should then be stopped and oral labetalol hydrochloride started. The effective intravenous dose is usually in the range of 50 to 200 mg. A total dose of up to 300 mg may be required in some patients.
Blood Pressure Monitoring
The blood pressure should be monitored during and after completion of the infusion or intravenous injections. Rapid or excessive falls in either systolic or diastolic blood pressure during intravenous treatment should be avoided. In patients with excessive systolic hypertension, the decrease in systolic pressure should be used as indicator of effectiveness in addition to the response of the diastolic pressure.
Initiation of Dosing with Labetalol Hydrochloride Tablets
Subsequent oral dosing with labetalol hydrochloride tablets should begin when it has been established that the supine diastolic blood pressure has begun to rise. The recommended initial dose is 200 mg, followed in 6 to 12 hours by an additional dose of 200 or 400 mg, depending on the blood pressure response. Thereafter, inpatient titration with labetalol hydrochloride tablets may proceed as follows:
- *
- If needed, the total daily dose may be given in three divided doses.
Inpatient Titration Instructions
Regimen
Daily Dose*
200 mg b.i.d.
400 mg
400 mg b.i.d.
800 mg
800 mg b.i.d.
1600 mg
1200 mg b.i.d.
2400 mg
While in the hospital, the dosage of labetalol hydrochloride tablets may be increased at 1 day intervals to achieve the desired blood pressure reduction.
For subsequent outpatient titration or maintenance dosing see Labetalol Hydrochloride Tablets Product Information DOSAGE AND ADMINISTRATION for additional recommendations.
Compatibility with commonly used intravenous fluids
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Labetalol hydrochloride injection was tested for compatibility with commonly used intravenous fluids at final concentrations of 1.25 mg to 3.75 mg labetalol hydrochloride per mL of the mixture. Labetalol hydrochloride injection was found to be compatible with and stable (for 24 hours refrigerated or at room temperature) in mixtures with the following solutions:
Ringers Injection, USP
Lactated Ringers Injection, USP
5% Dextrose and Ringers Injection
5% Lactated Ringers and 5% Dextrose Injection
5% Dextrose Injection, USP
0.9% Sodium Chloride Injection, USP
5% Dextrose and 0.2% Sodium Chloride Injection, USP
2.5% Dextrose and 0.45% Sodium Chloride Injection, USP
5% Dextrose and 0.9% Sodium Chloride Injection, USP
5% Dextrose and 0.33% Sodium Chloride Injection, USP
Labetalol hydrochloride injection was NOT compatible with 5% Sodium Bicarbonate Injection, USP. Care should be taken when administering alkaline drugs, including furosemide, in combination with labetalol. Compatibility should be assured prior to administering these drugs together.
-
HOW SUPPLIED
Labetalol hydrochloride injection, USP 5 mg/mL, is supplied in:
Unit of Sale Concentration NDC 0409-2339-34 20 mg/4 mL Bundle containing 10 cartons of 1 Single-dose Carpuject™ cartridge with Luer Lock for the Carpuject Syringe System (5 mg/mL) Instructions for Use of the Syringe Systems
Instructions for using the Carpuject Syringe are available with the reusable Carpuject Holder, List 2049-02.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] Protect from freezing. Protect syringe from light.
Note: To prevent needle-stick injuries, needles and blunt cannulas should not be recapped, purposely bent, or broken by hand.
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
LAB-1223-3.0
Revised: 05/2021
-
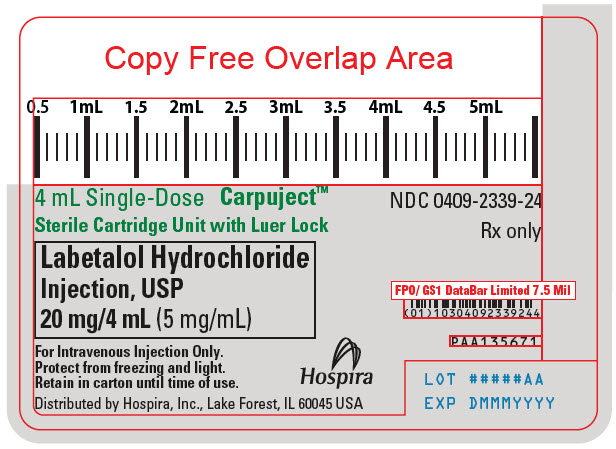
PRINCIPAL DISPLAY PANEL - 4 mL Cartridge Label
4 mL Single-Dose
Carpuject™Sterile Cartridge Unit with Luer Lock
Labetalol Hydrochloride
Injection, USP
20 mg/4 mL (5 mg/mL)For Intravenous Injection Only.
Protect from freezing and light.
Retain in carton until time of use.Hospira
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
NDC 0409-2339-24
Rx only
PAA135671
LOT #####AA
EXP DMMMYYYY

-
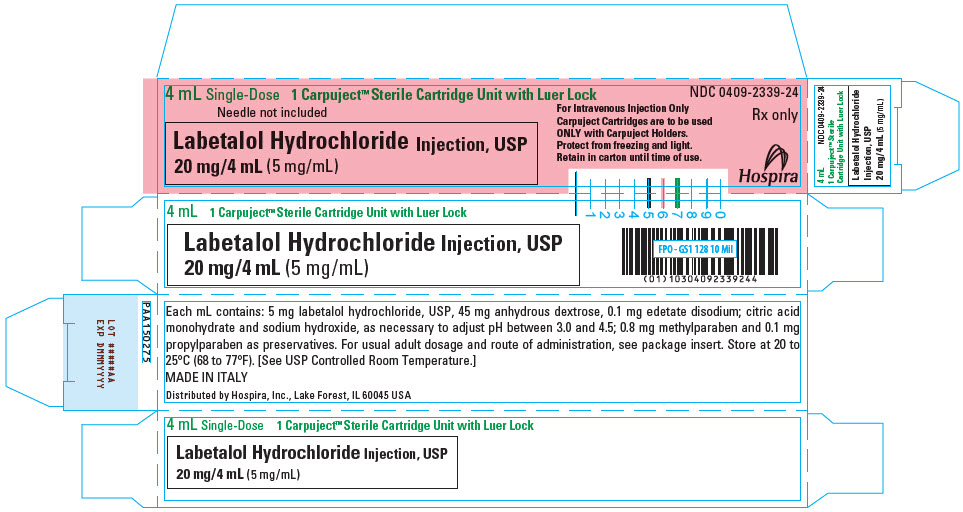
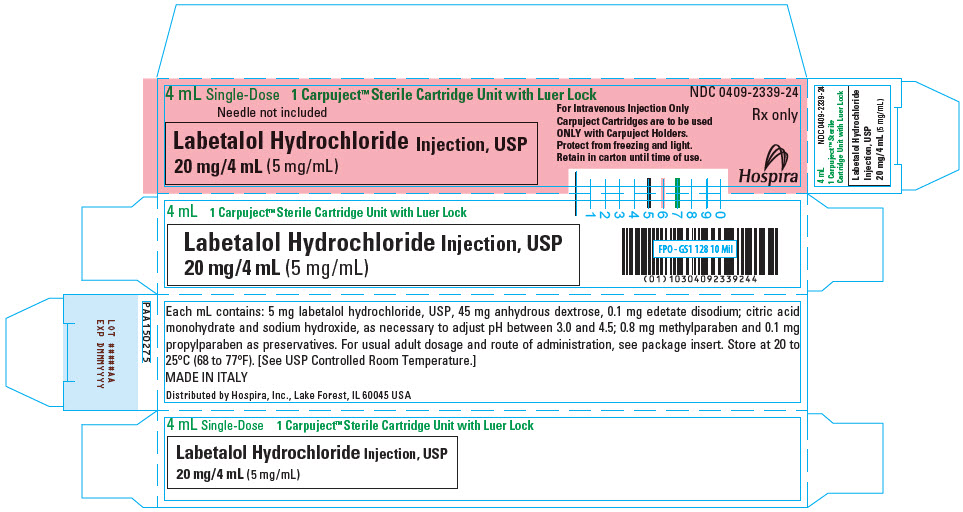
PRINCIPAL DISPLAY PANEL - 4 mL Cartridge Carton
4 mL Single-Dose
1 Carpuject™ Sterile Cartridge Unit with Luer LockNeedle not included
Labetalol Hydrochloride Injection, USP
20 mg/4 mL (5 mg/mL)For Intravenous Injection Only
Carpuject Cartridges are to be used
ONLY with Carpuject Holders.
Protect from freezing and light.
Retain in carton until time of use.NDC 0409-2339-24
Rx only
Hospira
-
INGREDIENTS AND APPEARANCE
LABETALOL HYDROCHLORIDE
labetalol hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0409-2339 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LABETALOL HYDROCHLORIDE (UNII: 1GEV3BAW9J) (LABETALOL - UNII:R5H8897N95) LABETALOL HYDROCHLORIDE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) 45 mg in 1 mL EDETATE DISODIUM (UNII: 7FLD91C86K) 0.1 mg in 1 mL METHYLPARABEN (UNII: A2I8C7HI9T) 0.8 mg in 1 mL PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.1 mg in 1 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0409-2339-34 10 in 1 PACKAGE 10/18/2005 1 1 in 1 CARTON 1 NDC:0409-2339-24 4 mL in 1 CARTRIDGE; Type 7: Separate Products Requiring Cross Labeling Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075239 10/18/2005 Labeler - Hospira, Inc. (141588017) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 030606222 ANALYSIS(0409-2339) , MANUFACTURE(0409-2339) , PACK(0409-2339) , LABEL(0409-2339)