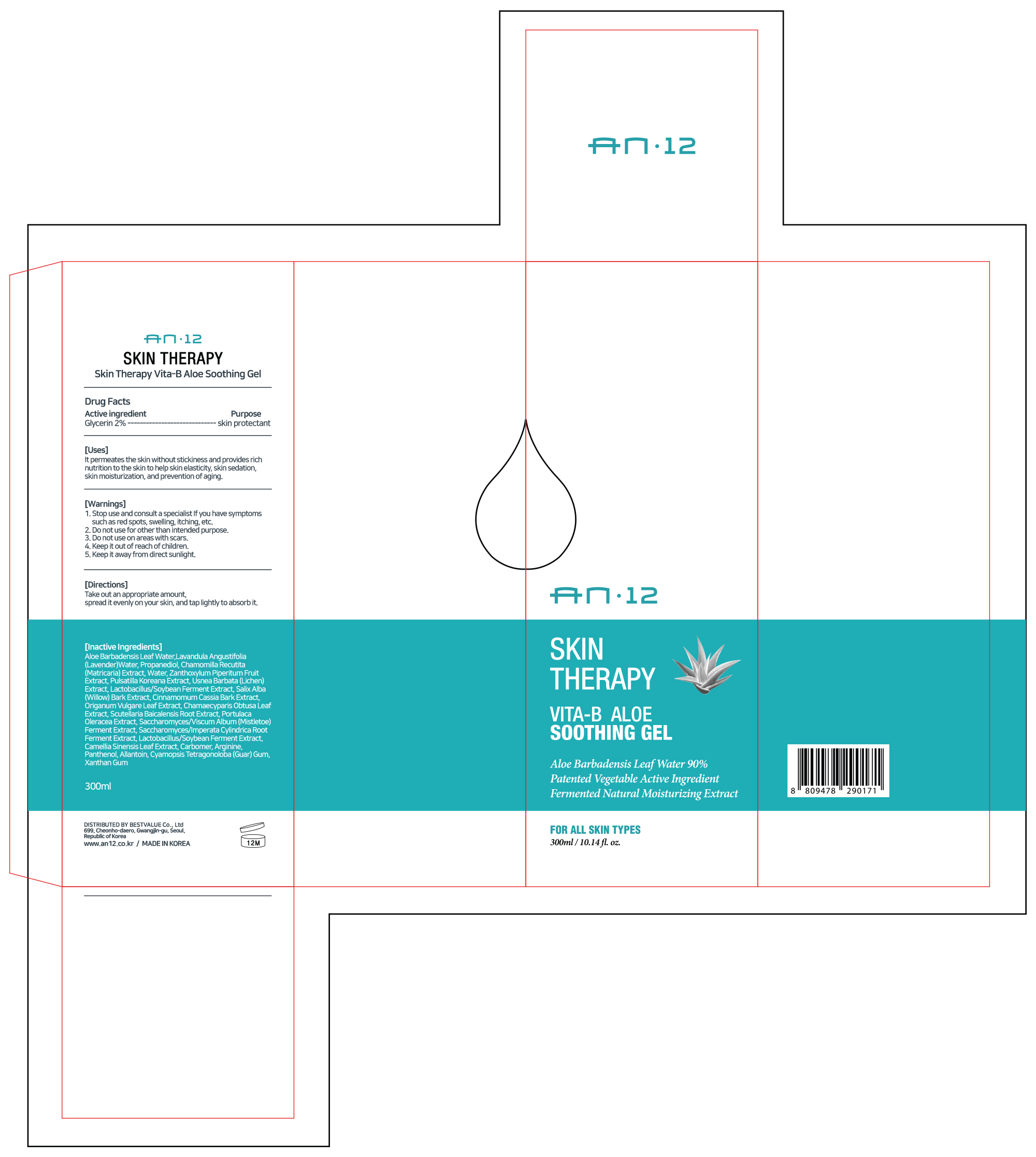
Label: AN12 SKIN THERAPY VITA-B ALOE SOOTHING GEL- glycerin gel
- NDC Code(s): 82385-104-01
- Packager: BESTVALUE Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Warnings
- Warnings
- Warnings
- Warnings
- Directions
-
Inactive Ingredients
Aloe Barbadensis Leaf Water, Lavandula Angustifolia (Lavender) Water, Propanediol,
Chamomilla Recutita (Matricaria) Extract, Water, Zanthoxylum Piperitum Fruit Extract,
Pulsatilla Koreana Extract, Usnea Barbata (Lichen) Extract,
Salix Alba (Willow) Bark Extract, Cinnamomum Cassia Bark Extract, Origanum Vulgare Leaf Extract,
Chamaecyparis Obtusa Leaf Extract, Scutellaria Baicalensis Root Extract,
Portulaca Oleracea Extract, Saccharomyces/Viscum Album (Mistletoe) Ferment Extract
Saccharomyces/Imperata Cylindrica Root Ferment Extract, Camellia Sinensis Leaf Extract, Carbomer, Arginine, Panthenol,
Allantoin, Cyamopsis Tetragonoloba (Guar) Gum, Xanthan Gum
- Display Panel Label
-
INGREDIENTS AND APPEARANCE
AN12 SKIN THERAPY VITA-B ALOE SOOTHING GEL
glycerin gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82385-104 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 2 g in 100 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) LAVENDER OIL (UNII: ZBP1YXW0H8) SALIX ALBA BARK (UNII: 205MXS71H7) OREGANO (UNII: 0E5AT8T16U) CHAMAECYPARIS OBTUSA LEAF (UNII: 7OL154J5XB) VISCUM ALBUM FRUITING TOP (UNII: BK9092J5MP) GREEN TEA LEAF (UNII: W2ZU1RY8B0) ARGININE (UNII: 94ZLA3W45F) WATER (UNII: 059QF0KO0R) PROPANEDIOL (UNII: 5965N8W85T) MATRICARIA CHAMOMILLA WHOLE (UNII: G0R4UBI2ZZ) SOYBEAN (UNII: L7HT8F1ZOD) LACTOBACILLUS ACIDOPHILUS (UNII: 1PRR1V42V5) PULSATILLA KOREANA ROOT (UNII: FY35I16MPL) USNEA BARBATA (UNII: D6DVA9TCAP) ZANTHOXYLUM PIPERITUM FRUIT PULP (UNII: 7PFC2VA251) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) GUAR GUM (UNII: E89I1637KE) IMPERATA CYLINDRICA ROOT (UNII: VYT2JA85NH) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) ALLANTOIN (UNII: 344S277G0Z) XANTHAN GUM (UNII: TTV12P4NEE) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82385-104-01 300 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 12/06/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/06/2021 Labeler - BESTVALUE Co., Ltd (694025785) Establishment Name Address ID/FEI Business Operations BESTVALUE Co., Ltd 694025785 manufacture(82385-104)