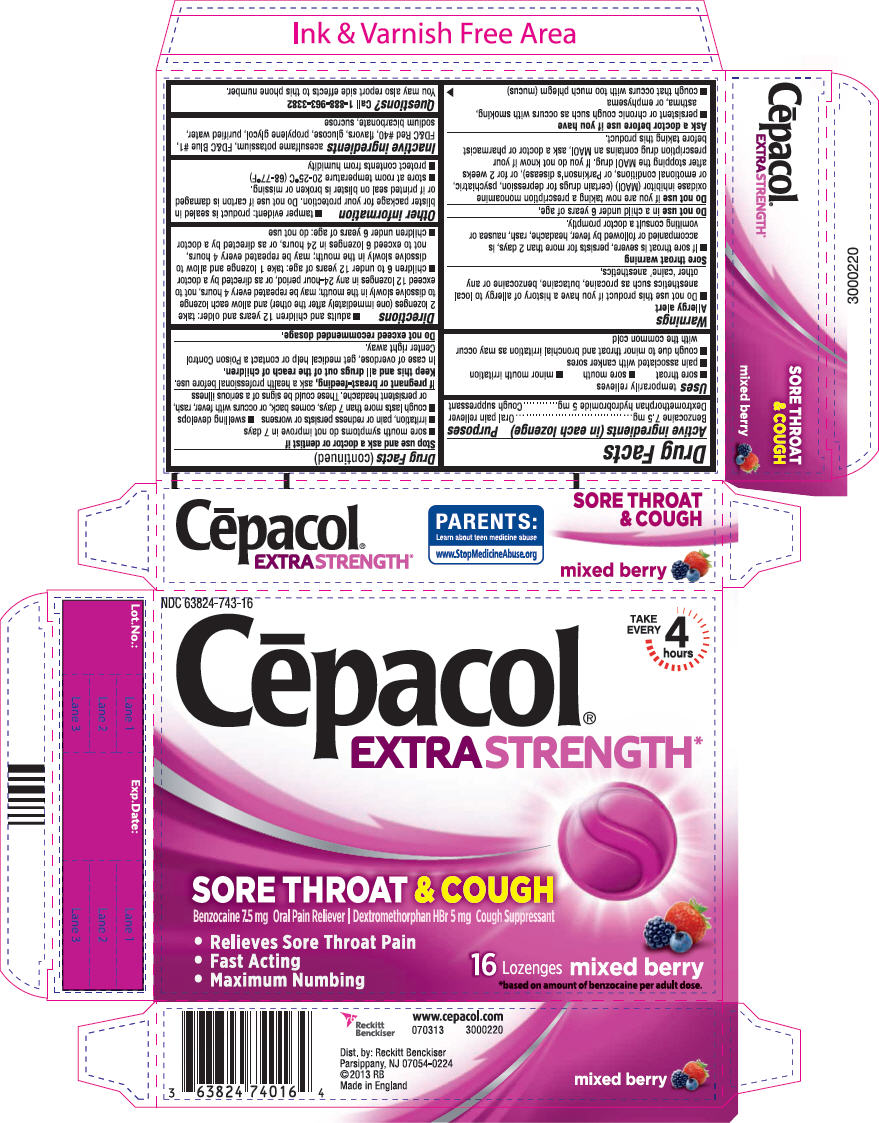
CEPACOL EXTRA STRENGTH SORE THROAT MIXED BERRY- benzocaine and dextromethorphan hydrobromide lozenge
RB Health (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Cēpacol
®
Extra Strength* Sore Throat mixed berry
Uses
temporarily relieves
- sore throat
- sore mouth
- minor mouth irritation
- pain associated with canker sores
- cough due to minor throat and bronchial irritation as may occur with the common cold
Warnings
Allergy alert
- Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other 'caine' anesthetics.
Sore throat warning
- If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea or vomiting consult a doctor promptly.
Do not usein a child under 6 years of age.
Do not useif you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
- cough that occurs with too much phlegm (mucus)
Stop use and ask a doctor or dentist if
- sore mouth symptoms do not improve in 7 days
- irritation, pain or redness persists or worsens
- swelling develops
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious illness
Directions
- adults and children 12 years and older: take 2 lozenges (one immediately after the other) and allow each lozenge to dissolve slowly in the mouth; may be repeated every 4 hours, not to exceed 12 lozenges in any 24-hour period, or as directed by a doctor
- children 6 to under 12 years of age: take 1 lozenge and allow to dissolve slowly in the mouth; may be repeated every 4 hours, not to exceed 6 lozenges in 24 hours, or as directed by a doctor
- children under 6 years of age: do not use
Other information
- tamper evident: product is sealed in blister package for your protection. Do not use if carton is damaged or if printed seal on blister is broken or missing.
- store at room temperature 20-25°C (68-77°F)
- protect contents from humidity
Inactive ingredients
acesulfame potassium, FD&C Blue #1, FD&C Red #40, flavors, glucose, propylene glycol, purified water, sodium bicarbonate, sucrose
PRINCIPAL DISPLAY PANEL - 16 Lozenge Carton
NDC 63824-743-16
Cēpacol
®
EXTRA STRENGTH*
TAKE
EVERY
4
hours
SORE THROAT & COUGH
Benzocaine 7.5 mg Oral Pain Reliever | Dextromethorphan HBr 5 mg Cough Suppressant
- Relieves Sore Throat Pain
- Fast Acting
- Maximum Numbing
16
Lozenges
mixed berry
*based on amount of benzocaine per adult dose.
| CEPACOL
EXTRA STRENGTH SORE THROAT MIXED BERRY
benzocaine and dextromethorphan hydrobromide lozenge |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - RB Health (US) LLC (081049410) |