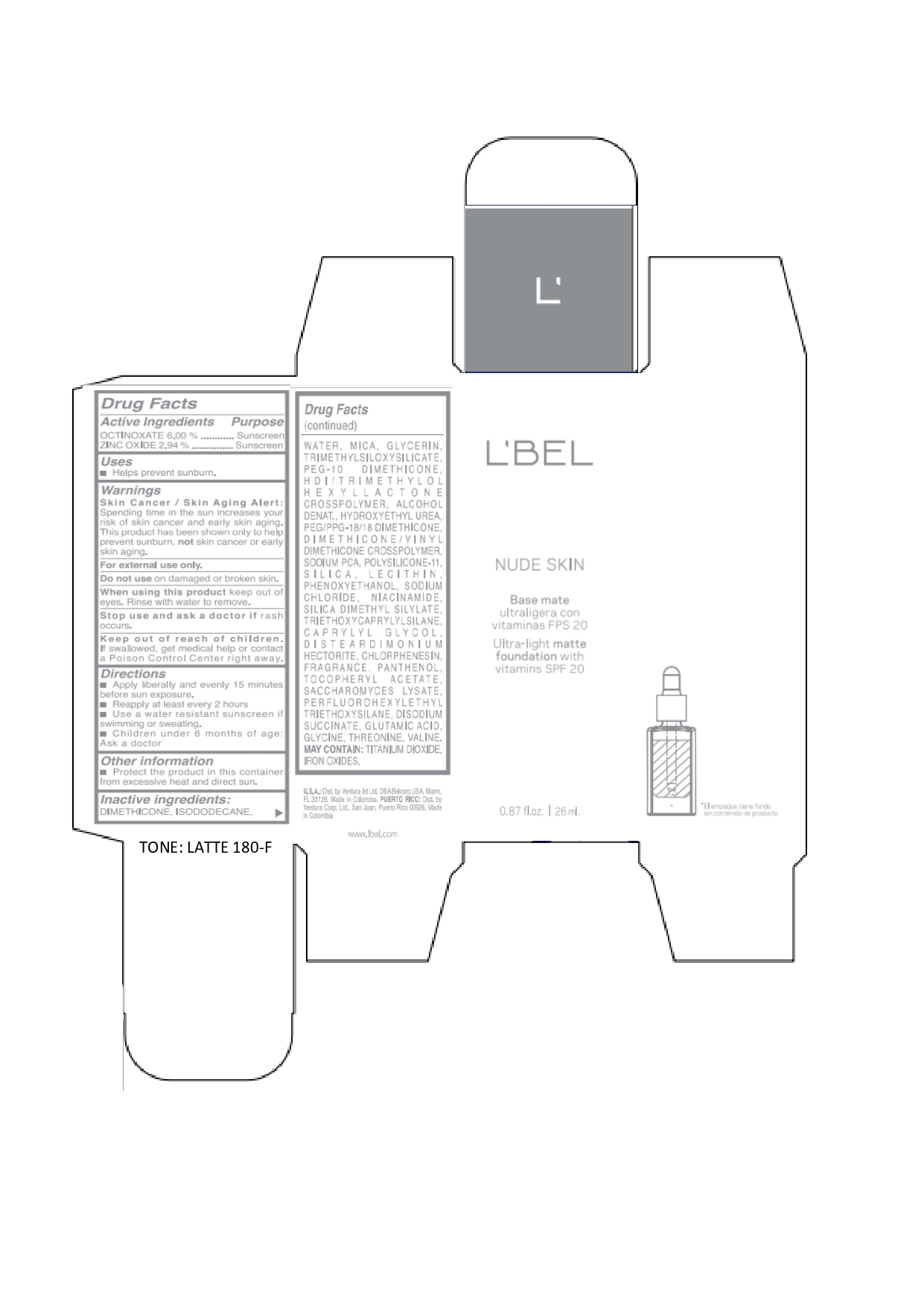
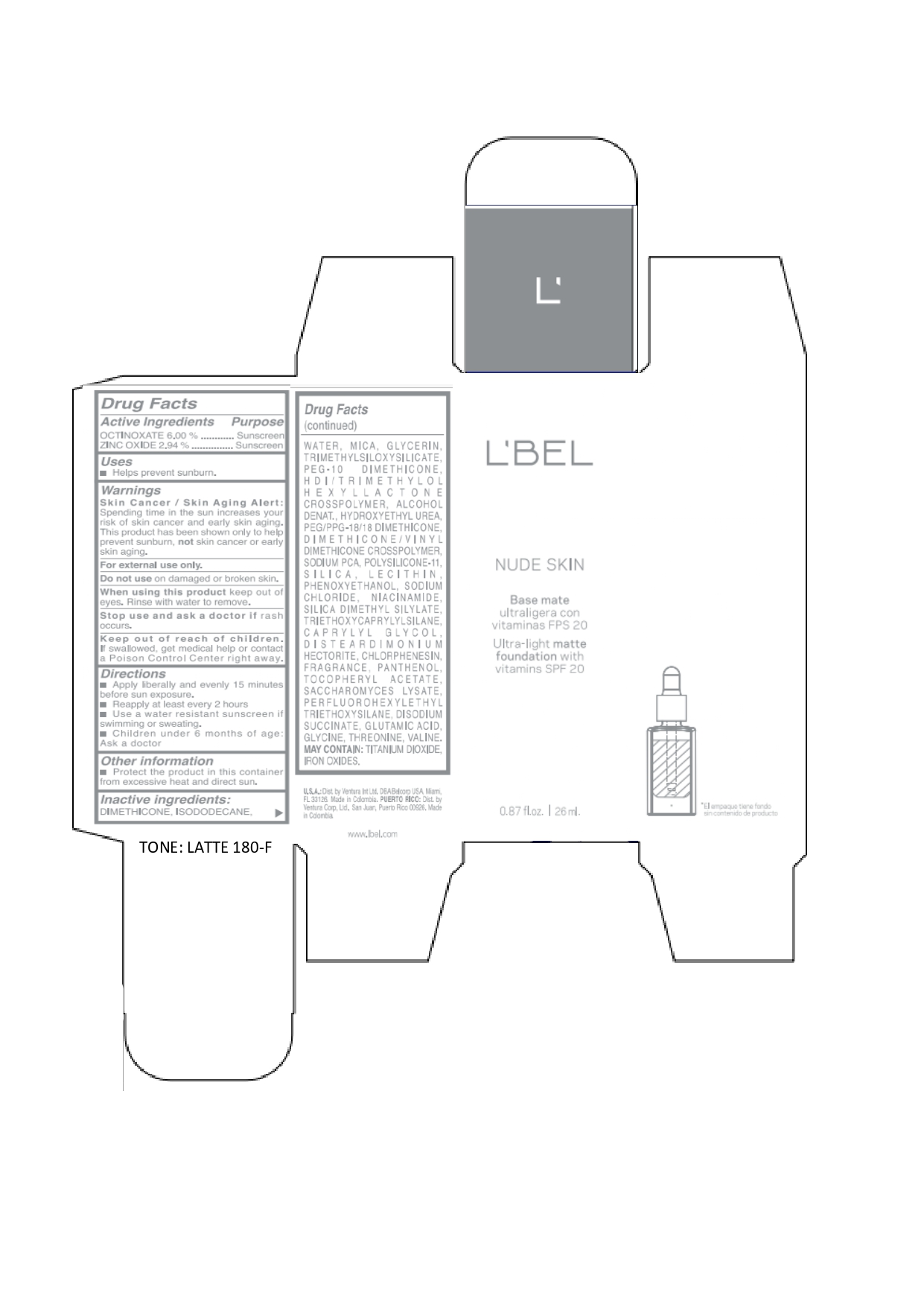
Label: LBEL NUDE SKIN ULTRA LIGHT MATTE FOUNDATION WITH VITAMINS SPF 20 LATTE 180-F- octinoxate, zinc oxide emulsion
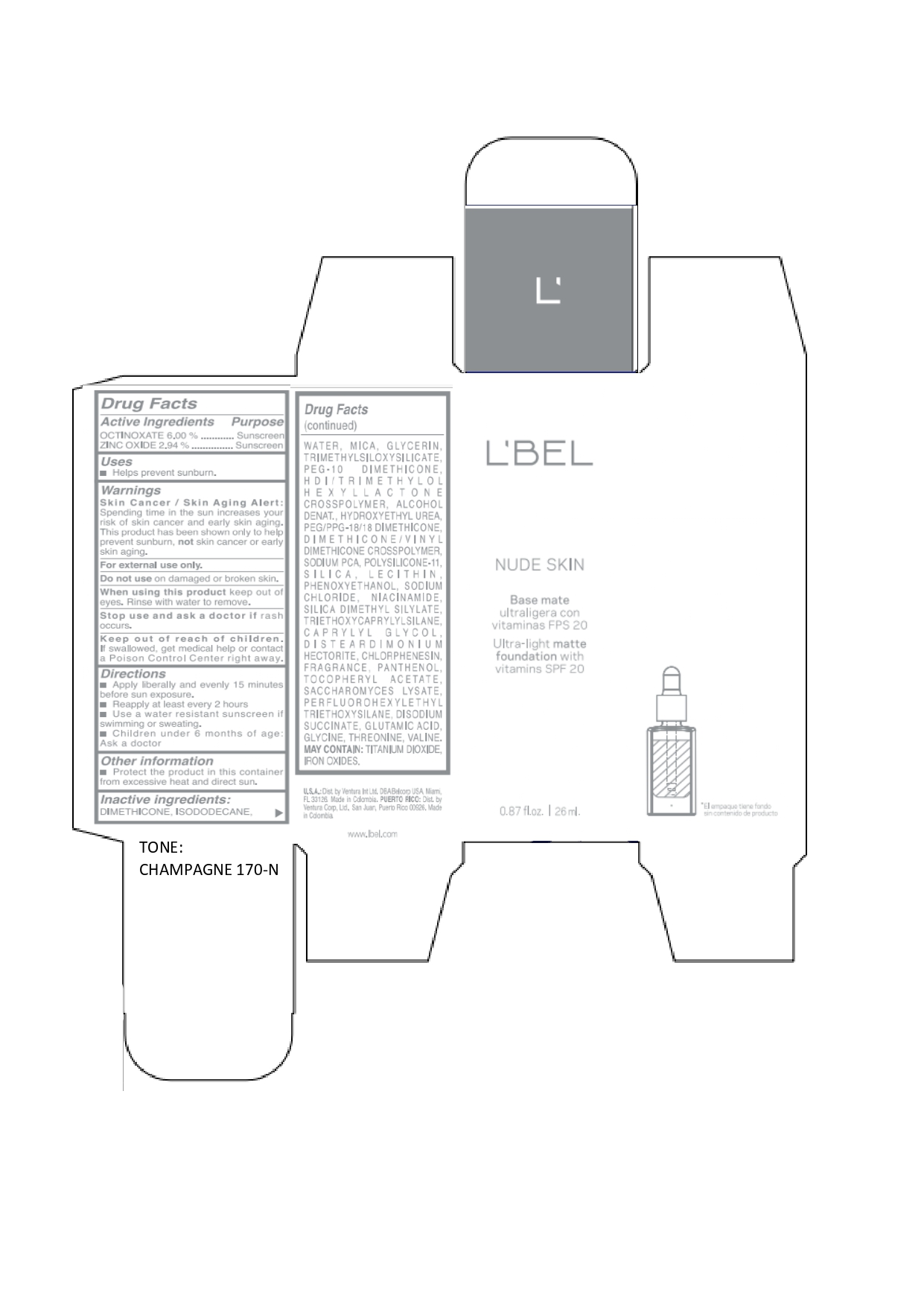
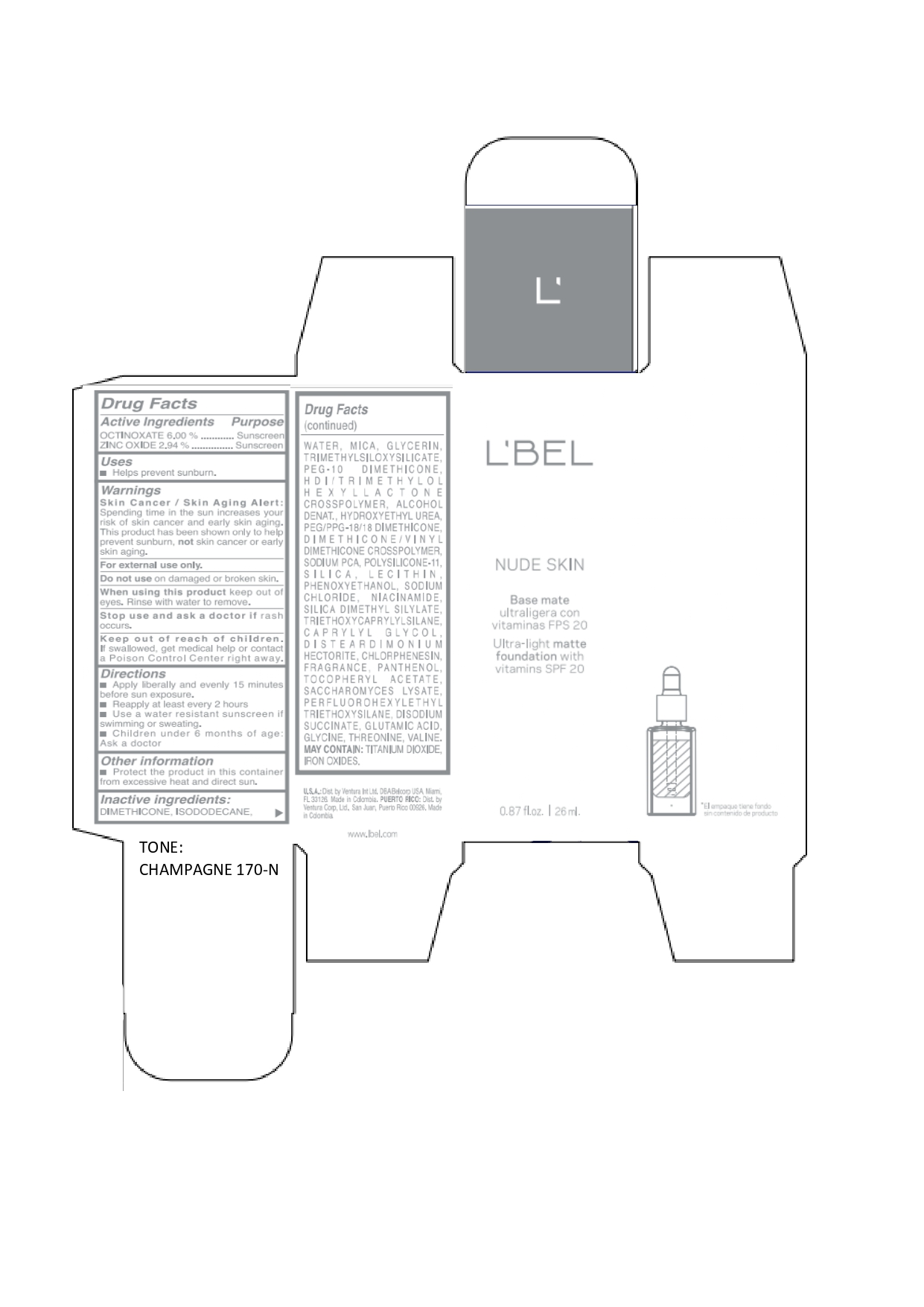
LBEL NUDE SKIN ULTRA LIGHT MATTE FOUNDATION WITH VITAMINS SPF 20 CHAMPAGNE 170-N- octinoxate, zinc oxide emulsion
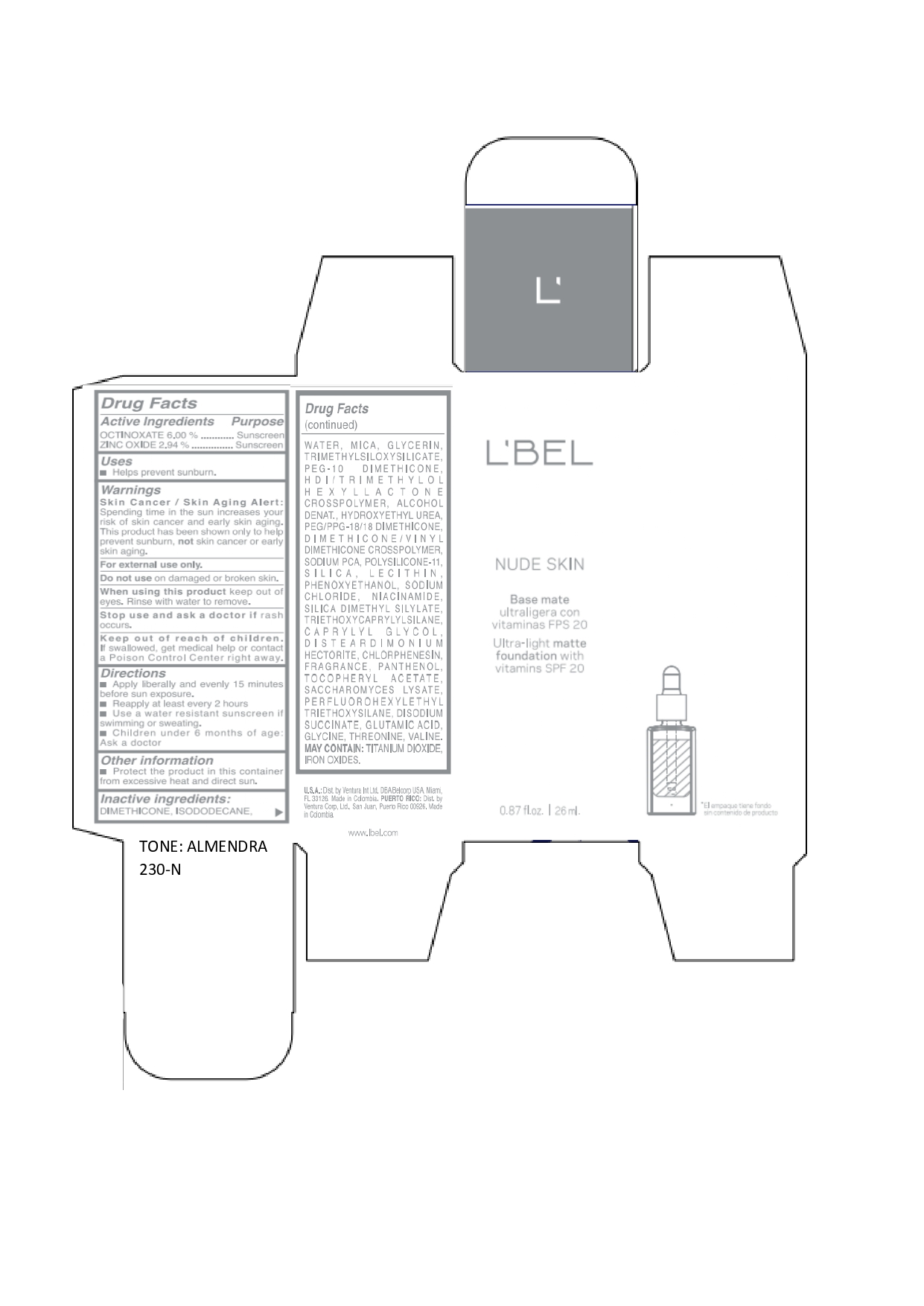
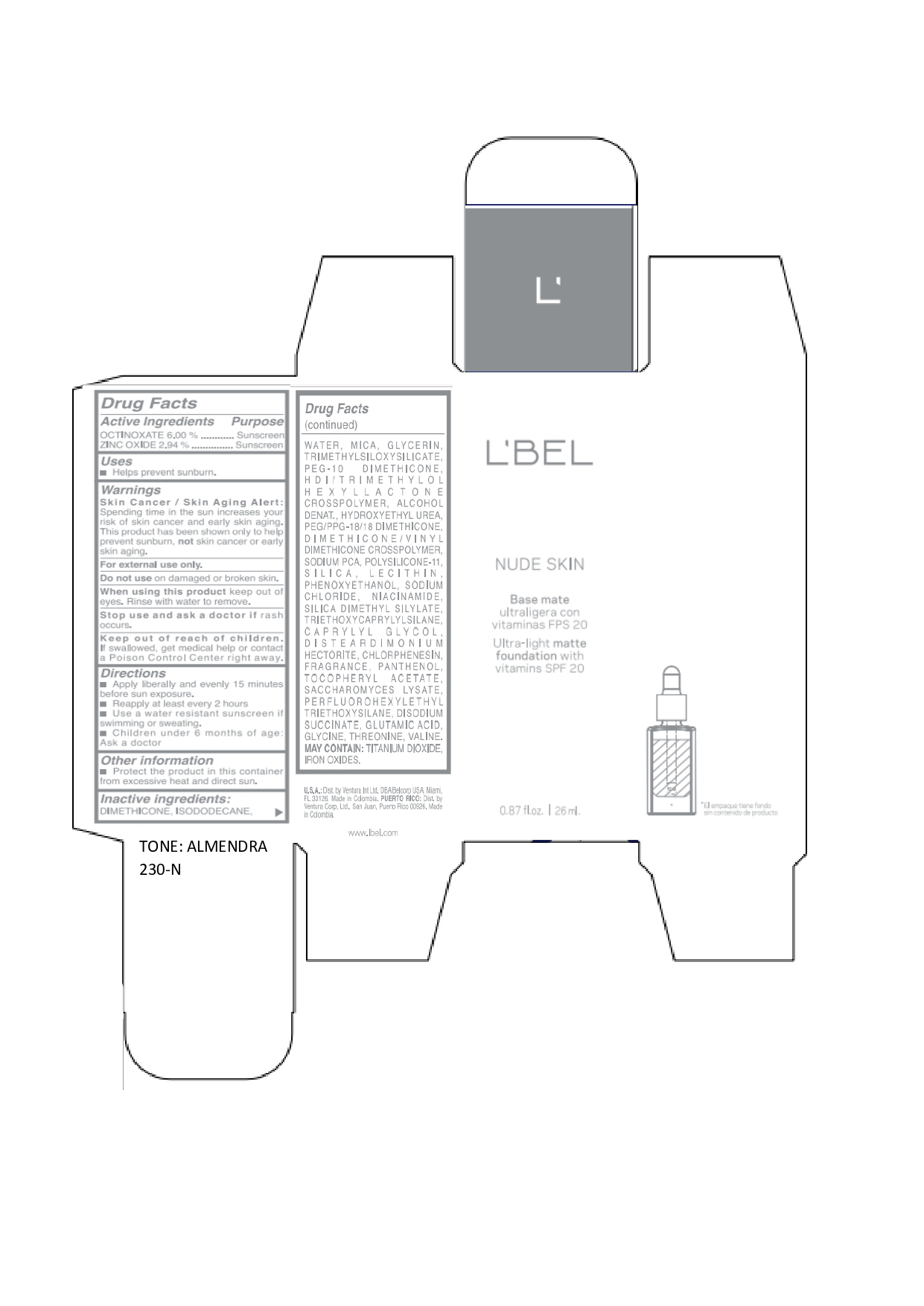
LBEL NUDE SKIN ULTRA LIGHT MATTE FOUNDATION WITH VITAMINS SPF 20 ALMENDRA 230-N- octinoxate, zinc oxide emulsion
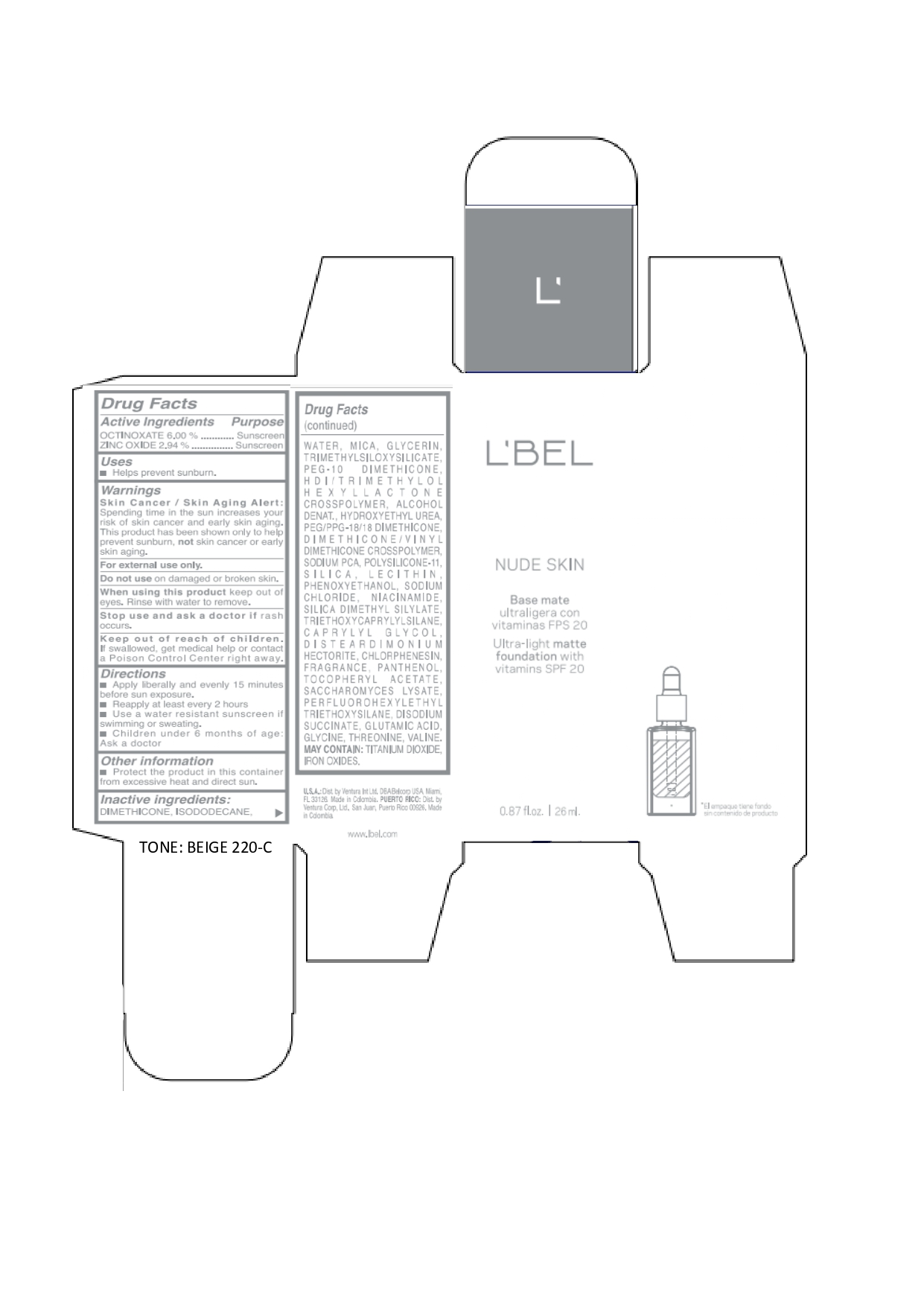
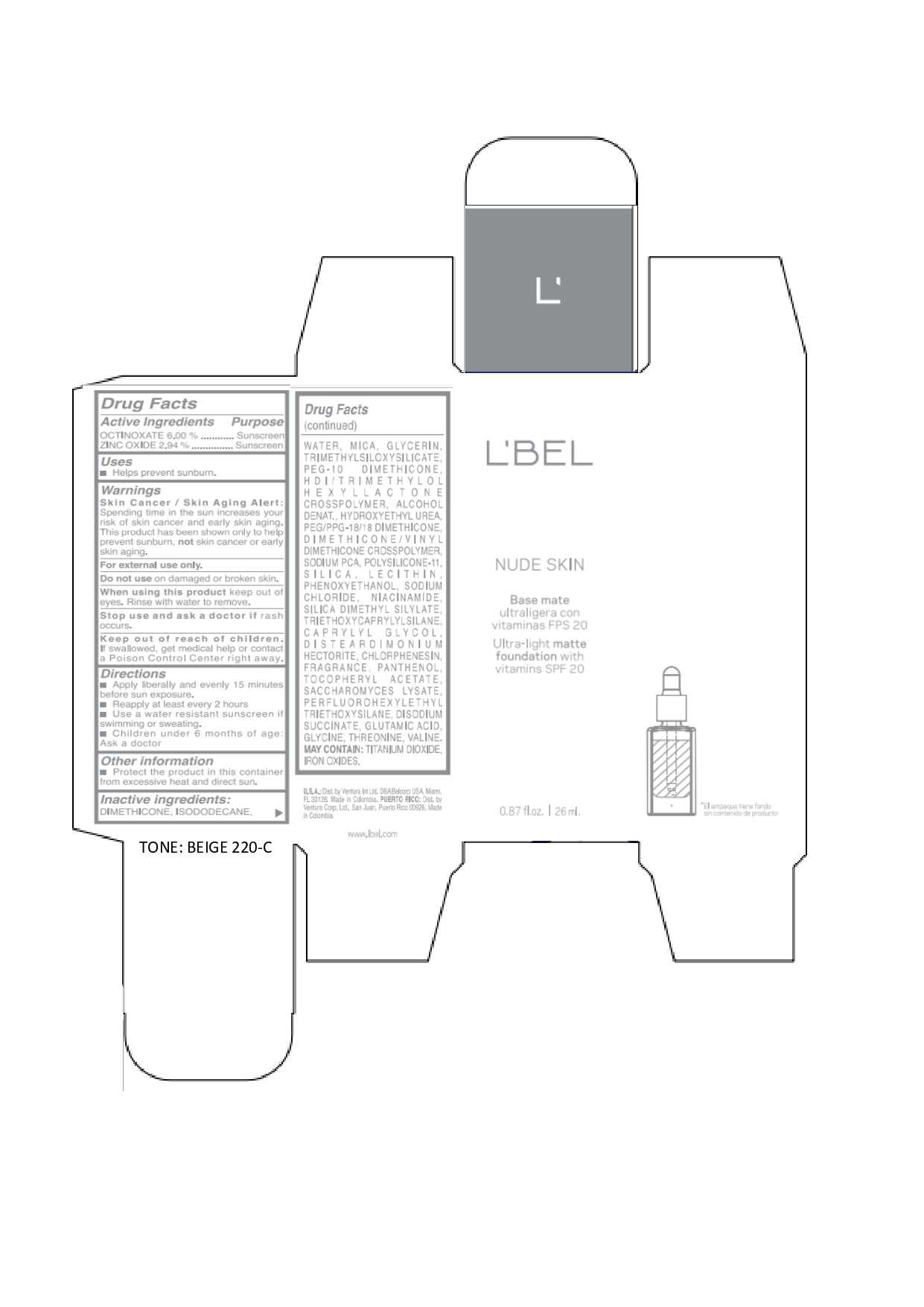
LBEL NUDE SKIN ULTRA LIGHT MATTE FOUNDATION WITH VITAMINS SPF 20 BEIGE 220-C- octinoxate, zinc oxide emulsion
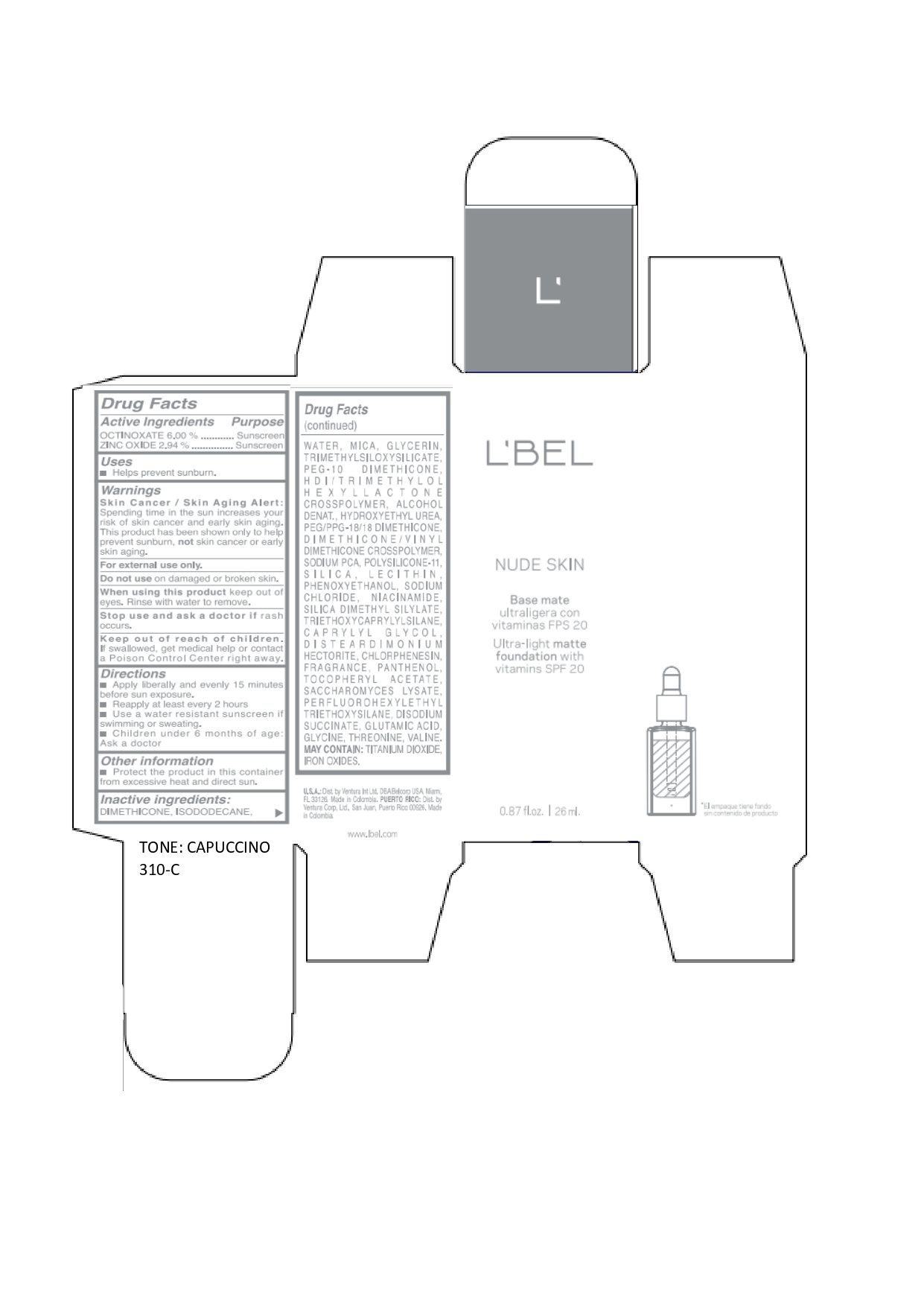
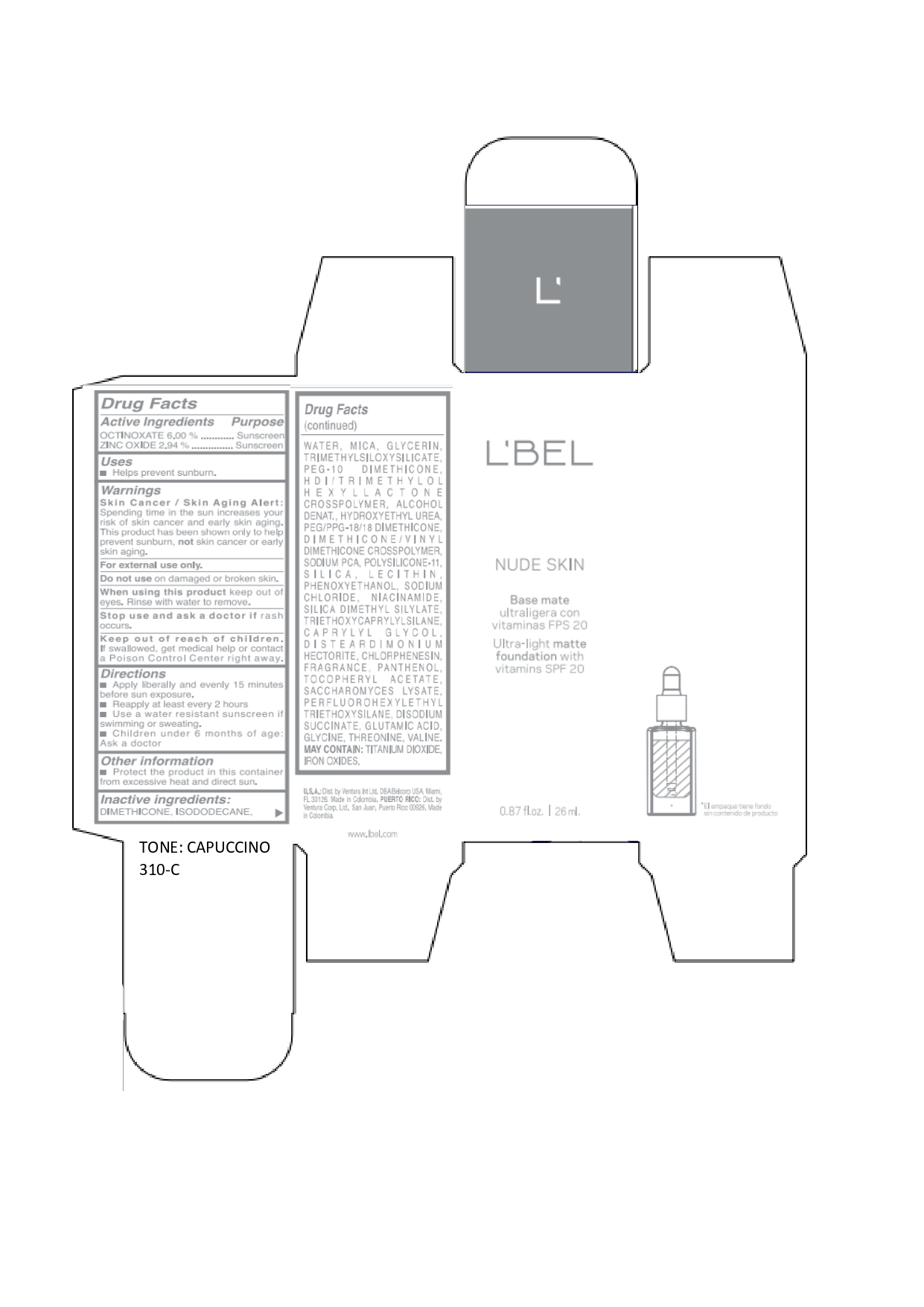
LBEL NUDE SKIN ULTRA LIGHT MATTE FOUNDATION WITH VITAMINS SPF 20 CAPUCCINO 310-C- octinoxate, zinc oxide emulsion
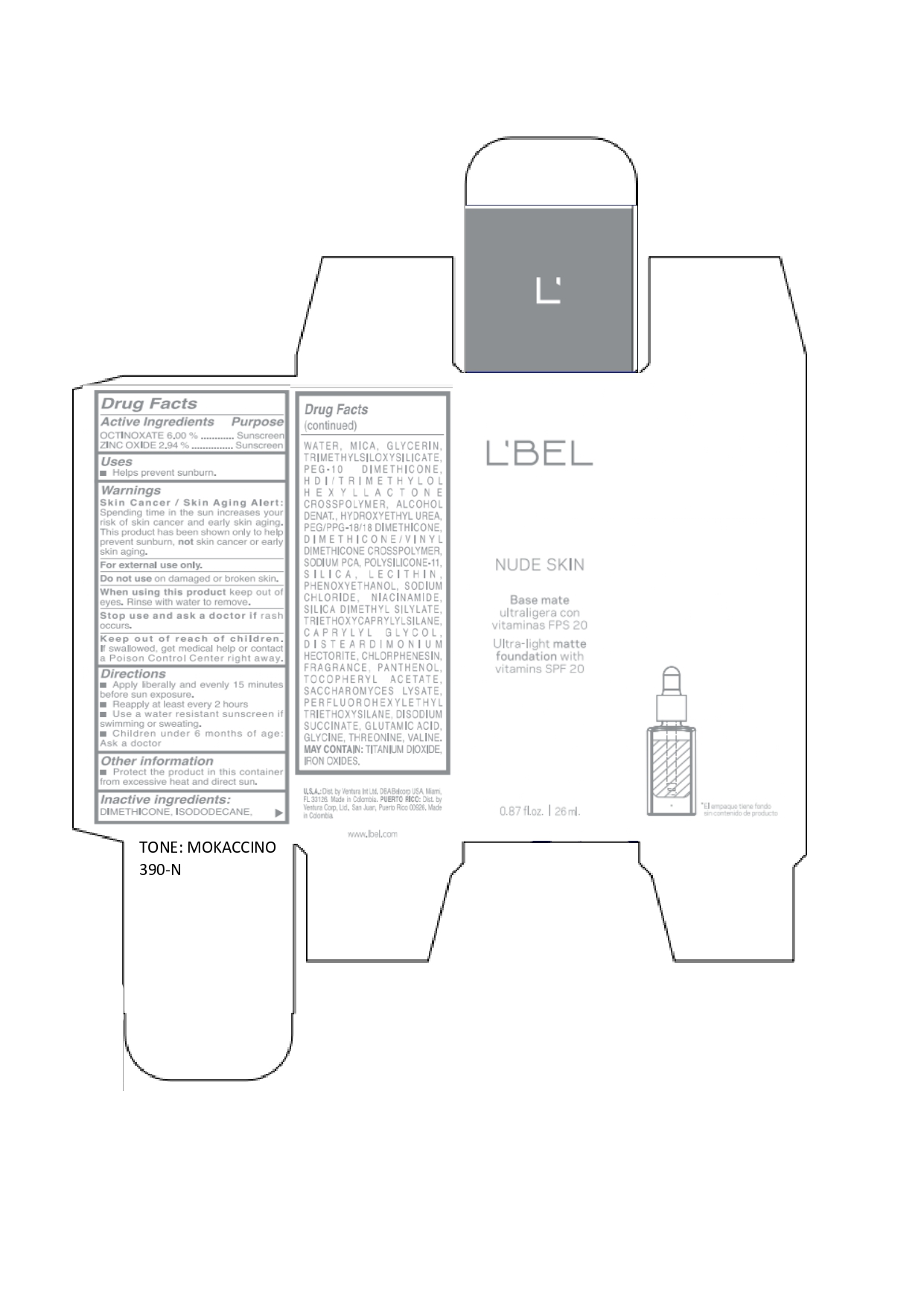
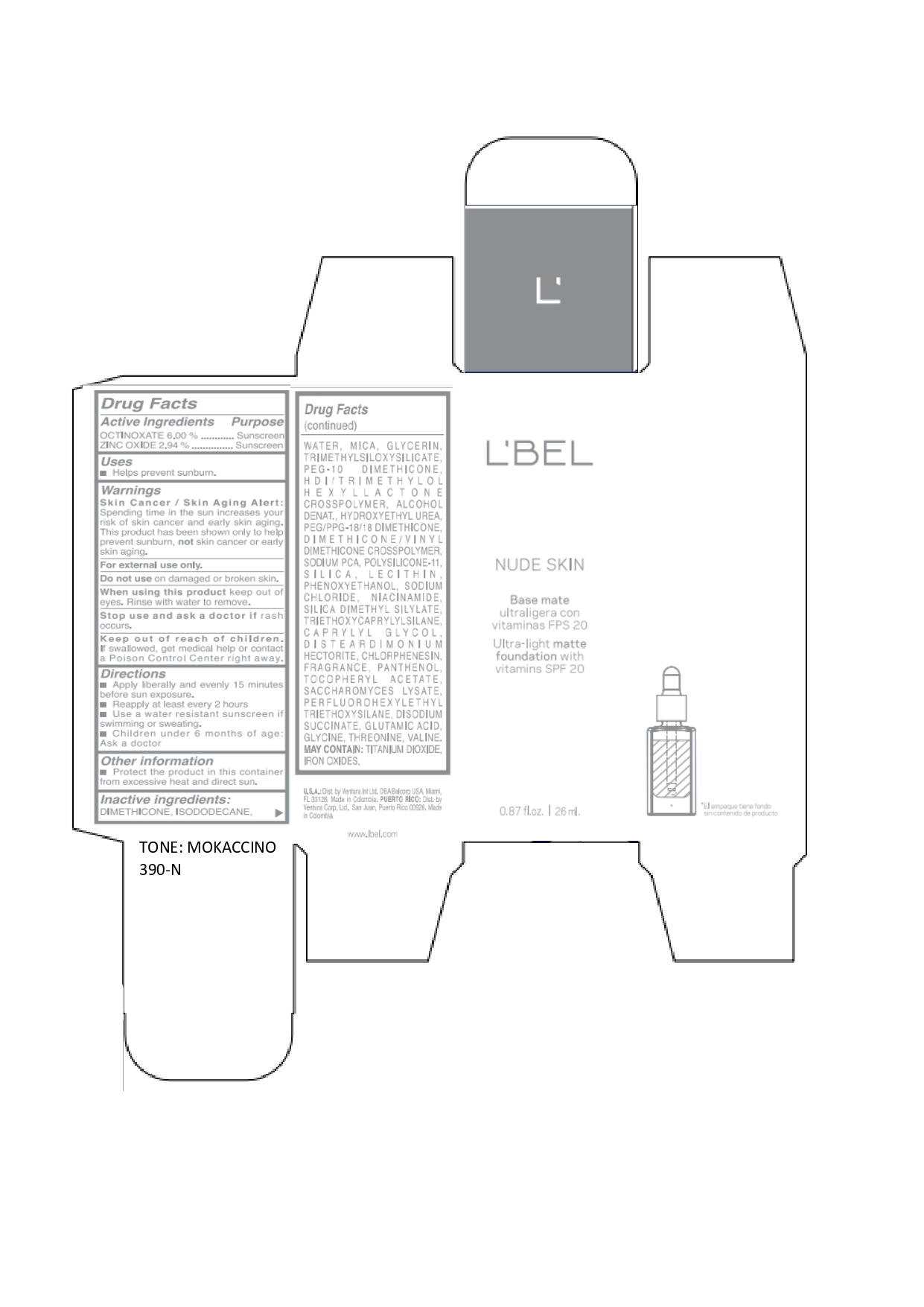
LBEL NUDE SKIN ULTRA LIGHT MATTE FOUNDATION WITH VITAMINS SPF 20 MOKACCINO 390-N- octinoxate, zinc oxide emulsion
-
NDC Code(s):
14141-017-01,
14141-018-01,
14141-019-01,
14141-020-01, view more14141-021-01, 14141-022-01
- Packager: Bel Star S.A.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 2, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
DIMETHICONE, ISODODECANE, WATER, MICA, GLYCERIN, TRIMETHYLSILOXYSILICATE, PEG-10 DIMETHICONE, HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, ALCOHOL DENAT., HYDROXYETHYL UREA, PEG/PPG-18/18 DIMETHICONE, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, SODIUM PCA, POLYSILICONE-11, SILICA, LECITHIN, PHENOXYETHANOL, SODIUM CHLORIDE, NIACINAMIDE, SILICA DIMETHYL SILYLATE, TRIETHOXYCAPRYLYLSILANE, CAPRYLYL GLYCOL, DISTEARDIMONIUM HECTORITE, CHLORPHENESIN, FRAGRANCE, PANTHENOL, TOCOPHERYL ACETATE, SACCHAROMYCES LYSATE, PERFLUOROHEXYLETHYL TRIETHOXYSILANE, DISODIUM SUCCINATE, GLUTAMIC ACID, GLYCINE, THREONINE, VALINE. MAY CONTAIN: TITANIUM DIOXIDE, IRON OXIDES.
- Company Information
- Product Packaging ALMENDRA 230-N
- Product Packaging BEIGE 220-C
- Product Packaging CAPUCCINO 310-C
- Product Packaging CHAMPAGNE 170-N
- Product Packaging LATTE 180-F
- Product Packaging MOKACCINO 390-N
-
INGREDIENTS AND APPEARANCE
LBEL NUDE SKIN ULTRA LIGHT MATTE FOUNDATION WITH VITAMINS SPF 20 LATTE 180-F
octinoxate, zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 29.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROXYETHYL UREA (UNII: MBQ7DDQ7AR) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SACCHAROMYCES LYSATE (UNII: R85W246Z1C) THREONINE (UNII: 2ZD004190S) WATER (UNII: 059QF0KO0R) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PANTHENOL (UNII: WV9CM0O67Z) VALINE (UNII: HG18B9YRS7) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) ALCOHOL (UNII: 3K9958V90M) SODIUM SUCCINATE ANHYDROUS (UNII: V8ZGC8ISR3) GLUTAMIC ACID (UNII: 3KX376GY7L) GLYCINE (UNII: TE7660XO1C) CHLORPHENESIN (UNII: I670DAL4SZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SODIUM CHLORIDE (UNII: 451W47IQ8X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) FERRIC OXIDE RED (UNII: 1K09F3G675) NIACINAMIDE (UNII: 25X51I8RD4) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) PHENOXYETHANOL (UNII: HIE492ZZ3T) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ISODODECANE (UNII: A8289P68Y2) MICA (UNII: V8A1AW0880) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-021-01 1 in 1 BOX 01/19/2022 1 26 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/19/2022 LBEL NUDE SKIN ULTRA LIGHT MATTE FOUNDATION WITH VITAMINS SPF 20 CHAMPAGNE 170-N
octinoxate, zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 29.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROXYETHYL UREA (UNII: MBQ7DDQ7AR) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SACCHAROMYCES LYSATE (UNII: R85W246Z1C) THREONINE (UNII: 2ZD004190S) WATER (UNII: 059QF0KO0R) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PANTHENOL (UNII: WV9CM0O67Z) VALINE (UNII: HG18B9YRS7) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) ALCOHOL (UNII: 3K9958V90M) SODIUM SUCCINATE ANHYDROUS (UNII: V8ZGC8ISR3) GLUTAMIC ACID (UNII: 3KX376GY7L) GLYCINE (UNII: TE7660XO1C) CHLORPHENESIN (UNII: I670DAL4SZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SODIUM CHLORIDE (UNII: 451W47IQ8X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) FERRIC OXIDE RED (UNII: 1K09F3G675) NIACINAMIDE (UNII: 25X51I8RD4) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) PHENOXYETHANOL (UNII: HIE492ZZ3T) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ISODODECANE (UNII: A8289P68Y2) MICA (UNII: V8A1AW0880) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-020-01 1 in 1 BOX 01/19/2022 1 26 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/19/2022 LBEL NUDE SKIN ULTRA LIGHT MATTE FOUNDATION WITH VITAMINS SPF 20 ALMENDRA 230-N
octinoxate, zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 29.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROXYETHYL UREA (UNII: MBQ7DDQ7AR) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SACCHAROMYCES LYSATE (UNII: R85W246Z1C) THREONINE (UNII: 2ZD004190S) WATER (UNII: 059QF0KO0R) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PANTHENOL (UNII: WV9CM0O67Z) VALINE (UNII: HG18B9YRS7) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) ALCOHOL (UNII: 3K9958V90M) SODIUM SUCCINATE ANHYDROUS (UNII: V8ZGC8ISR3) GLUTAMIC ACID (UNII: 3KX376GY7L) GLYCINE (UNII: TE7660XO1C) CHLORPHENESIN (UNII: I670DAL4SZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SODIUM CHLORIDE (UNII: 451W47IQ8X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) FERRIC OXIDE RED (UNII: 1K09F3G675) NIACINAMIDE (UNII: 25X51I8RD4) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) PHENOXYETHANOL (UNII: HIE492ZZ3T) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ISODODECANE (UNII: A8289P68Y2) MICA (UNII: V8A1AW0880) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-017-01 1 in 1 BOX 01/19/2022 1 26 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/19/2022 LBEL NUDE SKIN ULTRA LIGHT MATTE FOUNDATION WITH VITAMINS SPF 20 BEIGE 220-C
octinoxate, zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-018 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 29.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROXYETHYL UREA (UNII: MBQ7DDQ7AR) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SACCHAROMYCES LYSATE (UNII: R85W246Z1C) THREONINE (UNII: 2ZD004190S) WATER (UNII: 059QF0KO0R) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PANTHENOL (UNII: WV9CM0O67Z) VALINE (UNII: HG18B9YRS7) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) ALCOHOL (UNII: 3K9958V90M) SODIUM SUCCINATE ANHYDROUS (UNII: V8ZGC8ISR3) GLUTAMIC ACID (UNII: 3KX376GY7L) GLYCINE (UNII: TE7660XO1C) CHLORPHENESIN (UNII: I670DAL4SZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SODIUM CHLORIDE (UNII: 451W47IQ8X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) FERRIC OXIDE RED (UNII: 1K09F3G675) NIACINAMIDE (UNII: 25X51I8RD4) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) PHENOXYETHANOL (UNII: HIE492ZZ3T) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ISODODECANE (UNII: A8289P68Y2) MICA (UNII: V8A1AW0880) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-018-01 1 in 1 BOX 01/19/2022 1 26 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/19/2022 LBEL NUDE SKIN ULTRA LIGHT MATTE FOUNDATION WITH VITAMINS SPF 20 CAPUCCINO 310-C
octinoxate, zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-019 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 29.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROXYETHYL UREA (UNII: MBQ7DDQ7AR) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SACCHAROMYCES LYSATE (UNII: R85W246Z1C) THREONINE (UNII: 2ZD004190S) WATER (UNII: 059QF0KO0R) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PANTHENOL (UNII: WV9CM0O67Z) VALINE (UNII: HG18B9YRS7) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) ALCOHOL (UNII: 3K9958V90M) SODIUM SUCCINATE ANHYDROUS (UNII: V8ZGC8ISR3) GLUTAMIC ACID (UNII: 3KX376GY7L) GLYCINE (UNII: TE7660XO1C) CHLORPHENESIN (UNII: I670DAL4SZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SODIUM CHLORIDE (UNII: 451W47IQ8X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) FERRIC OXIDE RED (UNII: 1K09F3G675) NIACINAMIDE (UNII: 25X51I8RD4) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) PHENOXYETHANOL (UNII: HIE492ZZ3T) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ISODODECANE (UNII: A8289P68Y2) MICA (UNII: V8A1AW0880) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-019-01 1 in 1 BOX 01/19/2022 1 26 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/19/2022 LBEL NUDE SKIN ULTRA LIGHT MATTE FOUNDATION WITH VITAMINS SPF 20 MOKACCINO 390-N
octinoxate, zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-022 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 29.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROXYETHYL UREA (UNII: MBQ7DDQ7AR) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SACCHAROMYCES LYSATE (UNII: R85W246Z1C) THREONINE (UNII: 2ZD004190S) WATER (UNII: 059QF0KO0R) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PANTHENOL (UNII: WV9CM0O67Z) VALINE (UNII: HG18B9YRS7) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) ALCOHOL (UNII: 3K9958V90M) SODIUM SUCCINATE ANHYDROUS (UNII: V8ZGC8ISR3) GLUTAMIC ACID (UNII: 3KX376GY7L) GLYCINE (UNII: TE7660XO1C) CHLORPHENESIN (UNII: I670DAL4SZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SODIUM CHLORIDE (UNII: 451W47IQ8X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) FERRIC OXIDE RED (UNII: 1K09F3G675) NIACINAMIDE (UNII: 25X51I8RD4) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) PHENOXYETHANOL (UNII: HIE492ZZ3T) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ISODODECANE (UNII: A8289P68Y2) MICA (UNII: V8A1AW0880) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-022-01 1 in 1 BOX 01/19/2022 1 26 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/19/2022 Labeler - Bel Star S.A. (880160197)