RANITIDINE- ranitidine hydrochloride tablet, film coated
H-E-B
----------
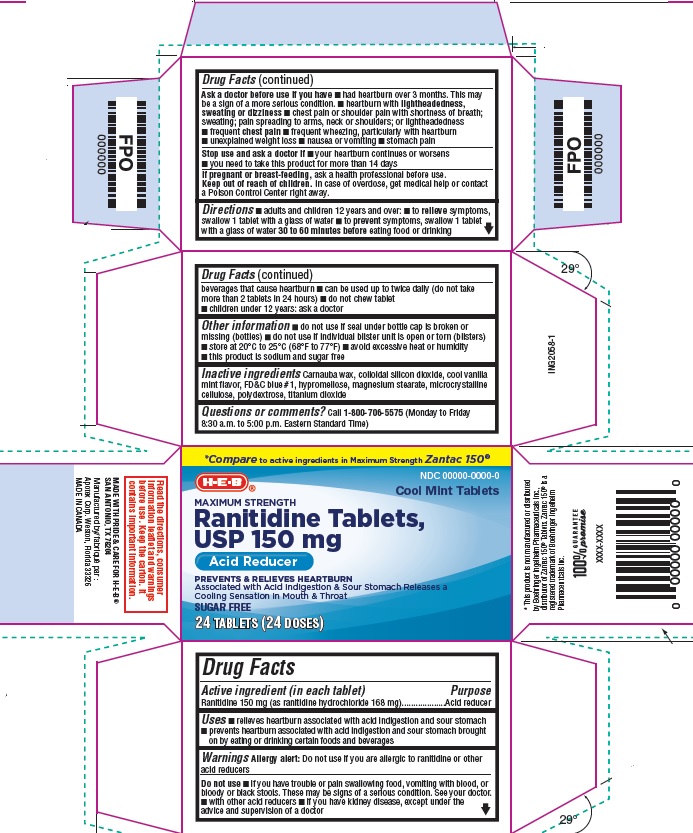
Drug Facts
Uses
- relieves heartburn associated with acid indigestion and sour stomach
- prevents heartburn associated with acid indigestion and sour stomach brought on by eating or drinking certain foods and beverages
Warnings
Allergy alert: Do not use if you are allergic to ranitidine or other acid reducers
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- with other acid reducers
- if you have kidney disease, except under the advice and supervision of a doctor
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
Directions
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water
- to prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating food or drinking beverages that cause heartburn
- can be used up to twice daily (do not take more than 2 tablets in 24 hours)
- Do not chew tablet
- children under 12 years: ask a doctor
Other information
- do not use if seal under bottle cap is broken or missing (bottles)
- do not use if individual blister unit is open or torn (blisters)
- store at 20°C to 25°C (68°F to 77°F)
- avoid excessive heat or humidity
- this product is sodium and sugar free
Inactive ingredients
carnauba wax, colloidal silicon dioxide, cool vanilla mint flavor, FD&C blue #1, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, titanium dioxide
Questions or Comments?
Call 1-800-706-5575 (Monday to Friday 8:30 a.m. – 5:00 p.m. Eastern Standard Time)
| RANITIDINE
ranitidine hydrochloride tablet, film coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - H-E-B (007924756) |
| Registrant - Apotex Inc. (209429182) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotex Inc | 209429182 | analysis(37808-303) , manufacture(37808-303) | |
Revised: 6/2022
Document Id: 37439e58-1128-8cd8-613a-aa3e4bb0f413
Set id: d1f39d44-9fed-e72c-9011-d153bbfce894
Version: 2
Effective Time: 20220614
H-E-B