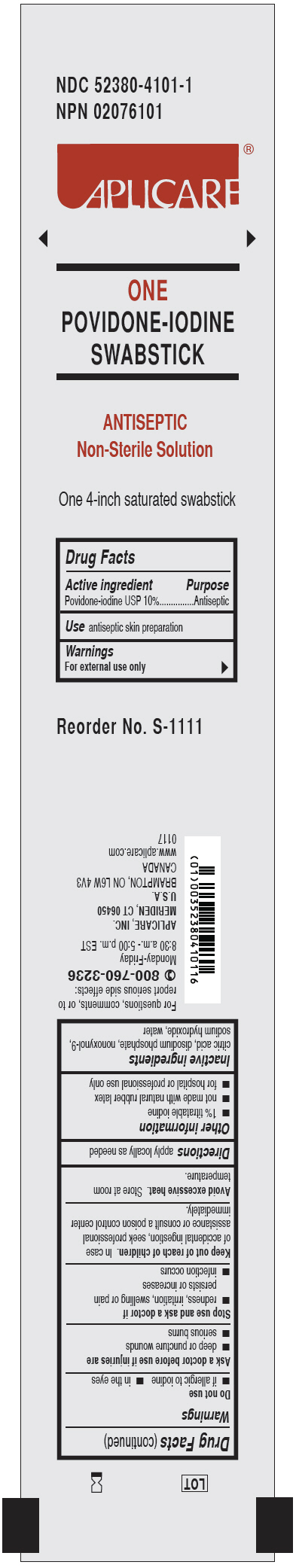
APLICARE POVIDONE IODINE SWABSTICK- povidone-iodine solution
Aplicare Products, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
4101 Aplicare Povidone Iodine Swabstick
Warnings
For external use only
Stop use and ask a doctor if
- • redness, irritation, swelling or pain persists or increases
- • infection occurs
Other information
- • 1% titratable iodine
- • not made with natural rubber latex
- • for hospital or professional use only
| APLICARE POVIDONE IODINE SWABSTICK
povidone-iodine solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Aplicare Products, LLC (081054904) |
| Registrant - Medline Industries, LP (025460908) |
Revised: 12/2021
Document Id: d22cbf80-f3ff-6447-e053-2995a90a035c
Set id: d1d9daeb-039e-4f28-8eec-6403c8ffb752
Version: 3
Effective Time: 20211202
Aplicare Products, LLC